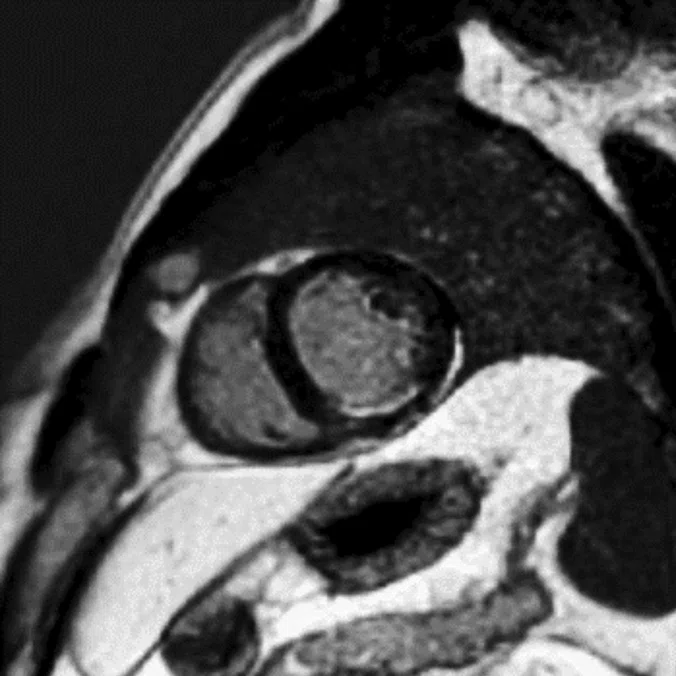
Hyper-intense signal difference between black blood and MDE defines area at risk. Image acquired on an OptimaTM MR450 1.5T system. Image Courtesy of Centro Cardiologico Monzino, Milano, Italy.
result


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
NEWS
Gadavist cleared as first contrast agent for cardiac MR
Gadavist cleared as first contrast agent for cardiac MR
Bayer’s Gadavist® is the first MR imaging agent approved by the FDA to assess myocardial perfusion, both stress and rest, and late gadolinium enhancement in adult patients with known or suspected coronary artery disease (CAD).
According to the company, the approval was based on two multinational, non- randomized, blinded-read Phase 3 studies of almost 1,000 adults with suspected or known CAD based on signs and symptoms. Nearly 800 of those patients were evaluated for efficacy.
The use of Gadavist® in a cardiac MR exam can provide the information clinicians need to assess perfusion and late gadolinium enhancement in less than one hour, which can help with patient management. Using the imaging agent, it is possible to get a clear picture of the blood supply serving the heart muscle, together with how well the blood flows or perfuses through the muscle at rest and during exercise.
Approximately 16.5 million Americans are affected by CAD, making it the most common form of heart disease.