1. Hulvershorn LA, Cullen KR, Francis M, Westlund M. Developmental Resting State Functional Connectivity for Clinicians. Curr Behav Neurosci Rep. 2014;1(3):161-169. doi:10.1007/s40473-014-0020-3.
1. Emory Sports Performance and Research Center, Flowery Branch, GA, USA
2. Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA, USA
1. Emory Sports Performance and Research Center, Flowery Branch, GA, USA
2. Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA, USA
1. Emory Sports Performance and Research Center, Flowery Branch, GA, USA
2. Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA, USA
3. Department of Neuroscience, University of Cincinnati School of Medicine, Cincinnati, OH, USA
1. Emory Sports Performance and Research Center, Flowery Branch, GA, USA
2. Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA, USA
4. Department of Diagnostic Radiology and Imaging Sciences, Division of Musculoskeletal Imaging, Emory University School of Medicine, Atlanta, GA, USA
1. Emory Sports Performance and Research Center, Flowery Branch, GA, USA
2. Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA, USA
1. Emory Sports Performance and Research Center, Flowery Branch, GA, USA
2. Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA, USA
5. Ohio Musculoskeletal & Neurological Institute; College of Health Sciences and Professions, Ohio University, Athens, OH, USA
1. Emory Sports Performance and Research Center, Flowery Branch, GA, USA
2. Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA, USA
6. Emory Sports Medicine Center, Atlanta, GA, USA
7. The Micheli Center for Sports Injury Prevention, Waltham, MA, USA
3. Anand M, Diekfuss JA, Slutsky-Ganesh AB, Grooms DR, Bonnette S, Barber Foss KD, DiCesare CA, Hunnicutt JL, Myer GD. Integrated 3D motion analysis with functional magnetic resonance neuroimaging to identify neural correlates of lower extremity movement. J Neurosci Methods. 2021;355:109108.
2. Rodriguez KM, Palmieri-Smith RM, Krishnan C. How does anterior cruciate ligament reconstruction affect the functioning of the brain and spinal cord? A systematic review with meta-analysis. Journal of Sport and Health Science. 2021;10(2):172-181.
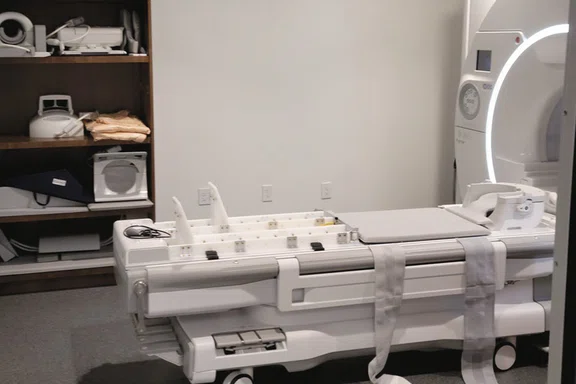
Figure 1.
SIGNA™ Premier at Emory SPARC. This scanner is fully dedicated towards research activities and is outfitted with a multi-camera, MR-Conditional, 3D motion analysis system for concurrent biomechanical assessment with fMRI.
4. Grooms DR, Diekfuss JA, Ellis JD, Yuan W, Dudley JA, Barber Foss KD, Thomas S, Altaye M, Haas L, Williams B, Lanier JM, Bridgewater K, Myer GD. A novel approach to evaluate brain activation for lower extremity motor control. Journal of Neuroimaging. 2019;29(5):580-588.
4. Grooms DR, Diekfuss JA, Ellis JD, Yuan W, Dudley JA, Barber Foss KD, Thomas S, Altaye M, Haas L, Williams B, Lanier JM, Bridgewater K, Myer GD. A novel approach to evaluate brain activation for lower extremity motor control. Journal of Neuroimaging. 2019;29(5):580-588.
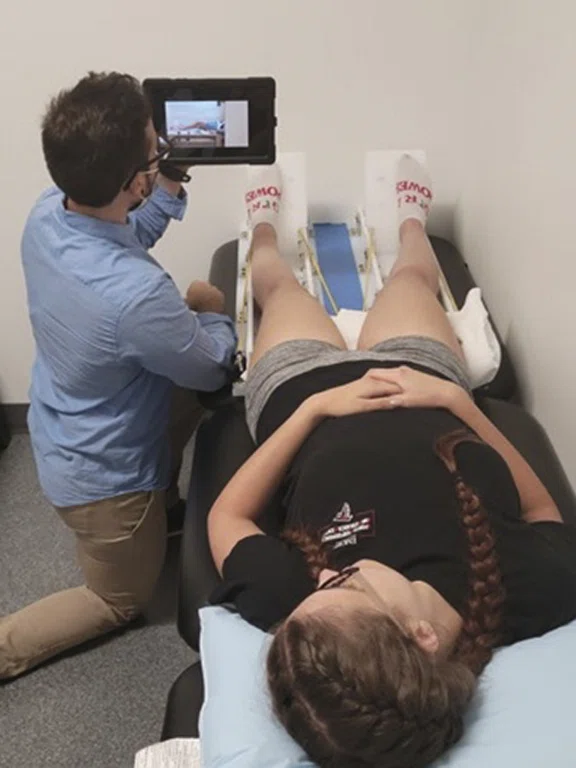
Figure 2.
Participant completing a ‘mock scanner’ training session prior to active brain scanning.
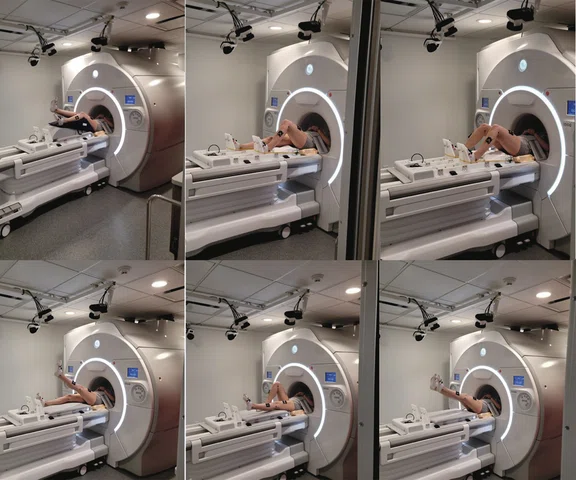
Figure 3.
Common lower extremity movements during active brain scanning (fMRI) at Emory SPARC. Top row (left to right): isolated knee flexion/extension, unilateral ‘leg press’ and bilateral ‘leg press’. Bottom row (left to right): single leg raises with contralateral limb extended, single leg raises with contralateral limb flexed and bilateral leg raises.
4. Grooms DR, Diekfuss JA, Ellis JD, Yuan W, Dudley JA, Barber
Foss KD, Thomas S, Altaye M, Haas L, Williams B, Lanier
JM, Bridgewater K, Myer GD. A novel approach to evaluate
brain activation for lower extremity motor control. Journal of
Neuroimaging. 2019;29(5):580-588.
5. Anand M, Diekfuss JA, Slutsky-Ganesh AB, Bonnette S,
Grooms DR, Myer GD. Graphical interface for automated
management of motion artifact within fMRI acquisitions:
INFOBAR. SoftwareX. 2020;12:100598.
4. Grooms DR, Diekfuss JA, Ellis JD, Yuan W, Dudley JA, Barber
Foss KD, Thomas S, Altaye M, Haas L, Williams B, Lanier
JM, Bridgewater K, Myer GD. A novel approach to evaluate
brain activation for lower extremity motor control. Journal of
Neuroimaging. 2019;29(5):580-588.
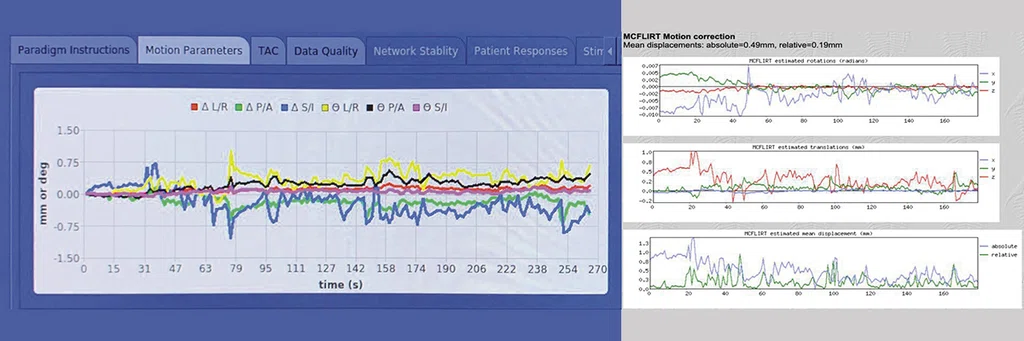
Figure 4.
(Left to right) Brainwave RT showing head motion in six movement planes in real time and a traditional post-processing output of head motion using FSL’s MCFLIRT with standard motion correction.
6. Diekfuss JA, Bonnette S, Hogg JA, Riehm C, Grooms DR, Singh H, Anand M, Slutsky AB, Wilkerson G, Myer GD. Practical training strategies to apply neuro-mechanistic motor learning principles to facilitate adaptations towards injury-resistant movement in youth. Journal of Science in Sport and Exercise. 2021;3:3-16.
7. Grooms DR, Kiefer AW, Riley MA, Ellis JD, Thomas S, Kitchen K, DiCesare CA, Bonnette S, Gadd B, Barber Foss KD, Yuan W, Silva P, Galloway R, Diekfuss JA, Leach J, Berz K, Myer GD. Brain-Behavior Mechanisms for the Transfer of Neuromuscular Training Adaptions to Simulated Sport: Initial Findings From the Train the Brain Project. J Sport Rehabil. 2018;27(5):1-5.
8. Diekfuss JA, Grooms DR, Hogg JA, Singh H, Slutsky AB, Bonnette S, Riehm C, Anand M, Nissen KS, Wilkerson G, Myer GD. Targeted application of motor learning theory to leverage youth neuroplasticity for enhanced injury-resistance and exercise performance: OPTIMAL PREP. Journal of Science in Sport and Exercise. 2021;3:56-65.
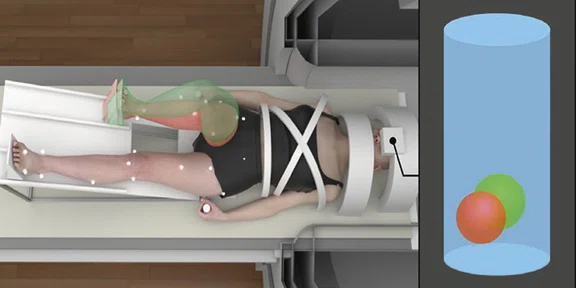
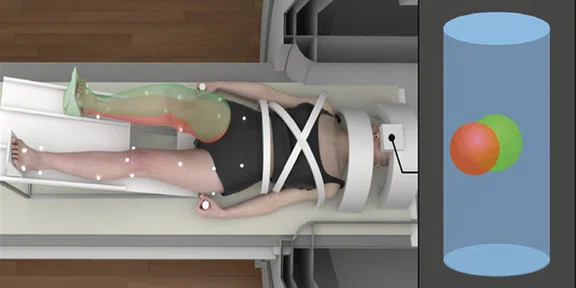
Figure 5.
Real-time biofeedback based on participant’s lower extremity biomechanics during brain fMRI. The green-tinted right limb demonstrates the target movement and corresponds to the position of the green ball. The red-tinted right limb depicts participant’s actual movement and corresponds to the position of the red ball, which is directly mapped to participant’s frontal and sagittal plane knee kinematics (positional-guided). Differences between the red and green balls on the 3D positional data can be used to quantify movement error concurrent with fMRI-derived brain activation metrics.
Figure 5.
Real-time biofeedback based on participant’s lower extremity biomechanics during brain fMRI. The green-tinted right limb demonstrates the target movement and corresponds to the position of the green ball. The red-tinted right limb depicts participant’s actual movement and corresponds to the position of the red ball, which is directly mapped to participant’s frontal and sagittal plane knee kinematics (positional-guided). Differences between the red and green balls on the 3D positional data can be used to quantify movement error concurrent with fMRI-derived brain activation metrics.
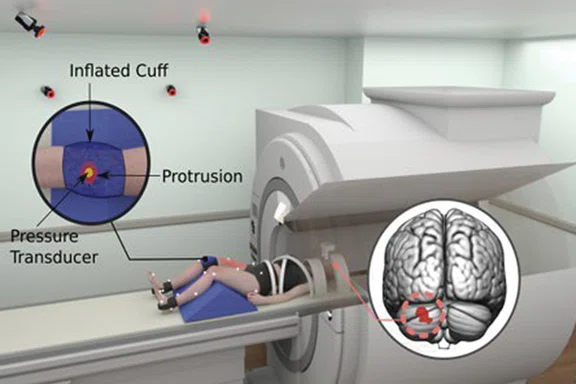
Figure 6.
(Top to bottom) Mechanical pain induction experiments for knee pain and low back pain concurrent with brain fMRI.
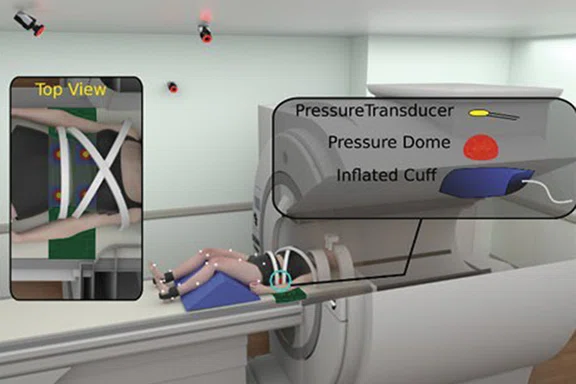
Figure 6.
(Top to bottom) Mechanical pain induction experiments for knee pain and low back pain concurrent with brain fMRI.
1. Emory Sports Performance and Research Center, Flowery Branch, GA, USA
2. Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA, USA
result


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
SPOTLIGHT
Measuring brain activity during dynamic movement: integrated fMRI and biomechanical methodologies
Measuring brain activity during dynamic movement: integrated fMRI and biomechanical methodologies
by Jed A. Diekfuss, PhD, Assistant, Professor1,2, Manish Anand, PhD, Research Associate 1,2 , Taylor Zuleger, BA/BS, doctoral student1,2,3, Shayla Warren, BS, Clinical Research Coordinator II1,2, HoWon Kim, MS, doctoral student 4, Alexis B. Slutsky-Ganesh, PhD, Post-doctoral Research Fellow1,2, Bryan R. Schlink, PhD, Post-doctoral Research Fellow1,2, Philip K. Wong, MD, radiologist4, Dustin R. Grooms, PhD, Associate Professor5, and Gregory D. Myer, Professor, Director of Emory SPARC 1,2,6,7
Functional magnetic resonance imaging (fMRI) has been the gold standard to measure brain activity for over three decades1. Despite being an indirect measure of neural activity, assessing the blood oxygen level dependent (BOLD) signal by means of fMRI has provided unprecedented insight into human brain function with excellent spatial resolution.
Functional magnetic resonance imaging (fMRI) has been the gold standard to measure brain activity for over three decades1. Despite being an indirect measure of neural activity, assessing the blood oxygen level dependent (BOLD) signal by means of fMRI has provided unprecedented insight into human brain function with excellent spatial resolution.
fMRI is particularly useful for mechanistic purposes to identify where and how the brain responds to a given variable or treatment. For instance, researchers have successfully identified important areas of the brain for various cognitive (attention, memory, etc.) and motor (finger movement, movement imagery, etc.) functions. However, there is one notable limitation of fMRI that has greatly impaired progression toward the discovery of how the brain contributes to motor control: head motion artifact. Specifically, to acquire high-quality neuroimaging data for quantitative analyses, the brain must remain near motionless during scanning. Thus, the field appeared to accept resting-state methods or basic finger tapping studies consisting of fine motor control as essentially the most dynamic motor-related paradigms possible with fMRI. However, the functionality of the central nervous system is critical for dynamic movement in numerous populations with and without movement disorders (e.g., athletes with anterior cruciate ligament [ACL] rupture and subsequent surgical reconstruction)2, warranting advanced methods to assess brain activity important for dynamic, gross lower extremity motor control.
Our team, consisting of human movement, neuroscience, biomechanics and motor control experts, have dedicated the past 15 years to overcome the technological, methodological and analytic limitations associated with head motion artifact to successfully employ fMRI during dynamic lower extremity motor tasks. Further, we have integrated MR-Conditional, 3D motion analysis systems to capture participants’ biomechanics concurrent with fMRI, all while maintaining the highest quality imaging data3. Herein we briefly summarize our current methods at Emory’s Sports Performance And Research Center (SPARC) that houses a SIGNA™ Premier 3.0T system – fully outfitted for dynamic lower extremity movement and biomechanical assessment concurrent with fMRI – to support our current NIH clinical trials and future scientific discoveries (Figure 1).
To utilize fMRI during lower extremity movement tasks at Emory SPARC, we prioritize the following prior to active data collection: 1) ‘mock scanner’ training with feedback and 2) movement restraint during scanning. The former is completed in an adjacent room to the scanner where our staff provides detailed instructions regarding head motion reduction, shows a standardized training video and has the participant practice the movements to be completed during the scanning session (Figure 2)4. Next, our research team secures numerous MR-Safe retroreflective markers on the participant’s lower extremities, which are ‘tracked’ by our motion analysis cameras once the participant enters the field of view (Figure 3). Once the participant is trained, prepped and ready to enter the MR room, considerable efforts are made to secure the participant with physical restraints to support the reduction of movement superior to the joint(s) of interest. Participants are positioned supine on the MR table with their head in the head coil surrounded with padding. Their legs are positioned in a custom apparatus with Velcro straps attached to the MR table to ‘secure’ the participant and reduce head motion. To minimize elbow flexion during the task and reduce accessory movement for relevant tasks, participants can hold on to vertical handlebars that were custom designed and secured to the side of the MR table at Emory SPARC. MR-Conditional fluidized positioners (Mölnlycke Health Care) that ‘mold’ to a given shape are also placed underneath the participant’s back, head, etc. for both reduction of extraneous motion and comfort4. Lastly, we have the participant complete practice movements in the MR while our research team monitors for excessive head motion through GE Healthcare’s Brainwave RT head motion tracking feature. If excess motion that would impair fMRI data quality is noted, we add more physical restraints and/or have the participant complete additional ‘mock scanner’ training.
Figure 3.
Common lower extremity movements during active brain scanning (fMRI) at Emory SPARC. Top row (left to right): isolated knee flexion/extension, unilateral ‘leg press’ and bilateral ‘leg press’. Bottom row (left to right): single leg raises with contralateral limb extended, single leg raises with contralateral limb flexed and bilateral leg raises.
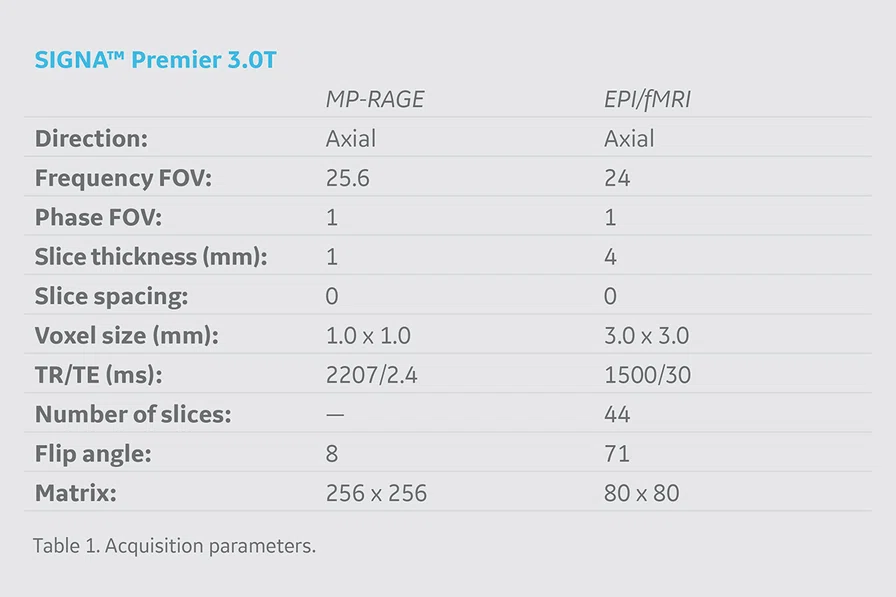
During the scanning session, we utilize the latest structural (MP-RAGE) and fMRI (echo-planar imaging [EPI]) sequences available on the SIGNA™ Premier. The specific acquisition parameters we utilize are detailed in Table 1. Following the MP-RAGE structural acquisition sequence, participants complete a series of EPI sequences aimed to isolate brain activity evoked from the movement of various lower extremity joints (e.g., knee, ankle and hip). Notably, we utilize HyperBand on the SIGNA™ Premier which has allowed us to collect an additional 45 volumes of data during the same acquisition period without compromising spatial resolution or data quality4.
As shown in Figure 3, the tasks we employ include, but are not limited to: isolated knee flexion/extension; unilateral multi-joint ankle, knee and hip flexion/extension against resistance; bilateral multi-joint ankle, knee and hip flexion/extension against resistance; single leg raises; and bilateral leg raises. The MR room is outfitted with eight MR-Conditional motion analysis cameras – mounted on tracks along the ceiling allowing them to ‘view’ desired movements of interest – which track joint positions concurrent with fMRI acquisition (Figures 1 and 3). Our 3D motion analysis system is intricately synced alongside our motor paradigms to allow for the precise quantification of brain activity related to specific kinematic movement variables (e.g., frontal plane knee range of motion). 3D motion analysis technologies concurrent with fMRI have transformed how we understand the neural contributions to dynamic motor control; however, our integrated methods may also divert a participant away from head motion reduction and toward biomechanical performance, thus we continue to supplement our prior methods with real-time data monitoring.
The SIGNA™ Premier includes an option for Brainwave RT, a unique and highly supportive feature that allows us to visualize head motion in realtime. Brainwave RT provides data in all six movement planes during fMRI acquisition (Figure 4). The real-time output reflects traditional outputs provided by analytic software packages after scanning is completed via standard pre/post-processing steps used for fMRI research purposes (e.g., MCFLIRT). Brainwave RT uniquely informs our research team to terminate a scan before its completion, readjust the participant, provide them reminders about head motion and/or enhance the physical restraints if head motion exceeds a threshold that would impair data quality (e.g., >1.25 mm of absolute motion or highly task-correlated motion). The team greatly values the head motion feature in Brainwave RT to support real-time quantitative data quality decision-making. This removes much of the guesswork to determine data quality while a participant is inside the scanner and avoids bad or lost data for research projects. In addition, Emory SPARC has developed numerous in-house data analytic pipelines and software tools to support the batch execution, quality assurance and ultimate removal of head motion artifact within our fMRI datasets (e.g., Interface for Batch processing fMRI datasets using ICA-AROMA [INFOBAR])5. Importantly, across all our paradigms, we robustly limit participants to <0.50 mm of absolute head motion with minimal task-correlated motion4. The systematic approach our team employs prior, during and post data acquisition ensures that reliable, high-quality fMRI data is achieved during dynamic motor control.
In conclusion, our team strives to bridge neuroscience with biomechanics to develop prevention, rehabilitation and performance techniques that promote adaptive neuroplasticity and injury-resistant movement6-8. Notably, our team is developing realtime biofeedback and virtual reality scenarios that will seamlessly integrate with our current methods to optimize the cognitive-perceptual realism of our testing paradigms (Figure 5). Further, we have adapted our fMRI motor paradigms to support advanced ‘movement-evoked pain’ methodologies to isolate central drivers of pain and fear in those with musculoskeletal movement disorders (e.g., chronic low back pain, patellofemoral pain; see Figure 6). Specifically, our team custom designs passive and active mechanical-based devices made from various MR-Conditional materials that are additively integrated with the SIGNA™ Premier system (e.g., 3D printed plastic screws, inflatable rubber ‘bladders’ to induce movement). Collectively our team is excited for the continued integration of the SIGNA™Premier with biomechanical, noxious stimulation, virtual reality, real-time bio/neurofeedback and fMRI methods and technologies of the future.
Figure 5.
Real-time biofeedback based on participant’s lower extremity biomechanics during brain fMRI. The green-tinted right limb demonstrates the target movement and corresponds to the position of the green ball. The red-tinted right limb depicts participant’s actual movement and corresponds to the position of the red ball, which is directly mapped to participant’s frontal and sagittal plane knee kinematics (positional-guided). Differences between the red and green balls on the 3D positional data can be used to quantify movement error concurrent with fMRI-derived brain activation metrics.