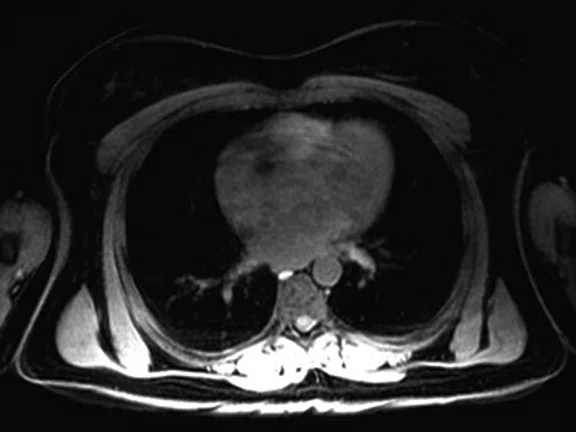
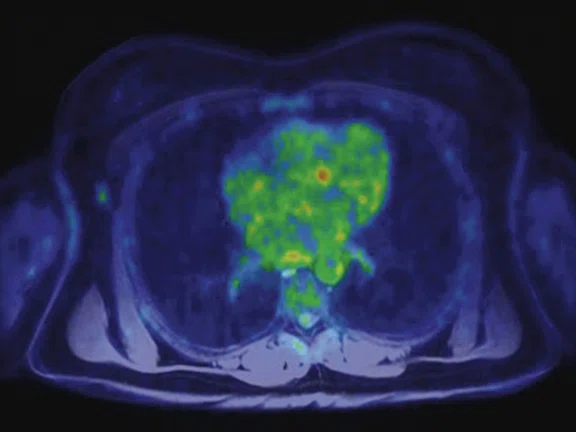
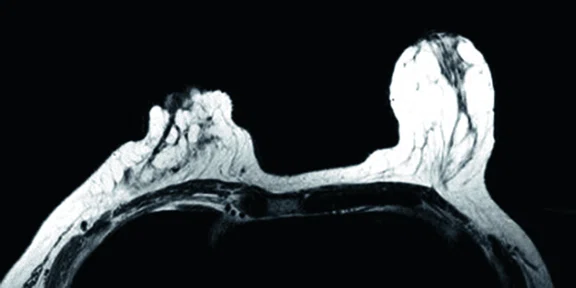
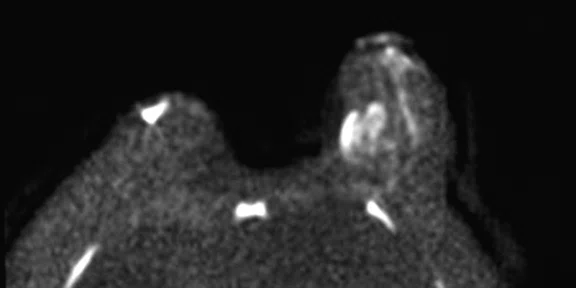
A
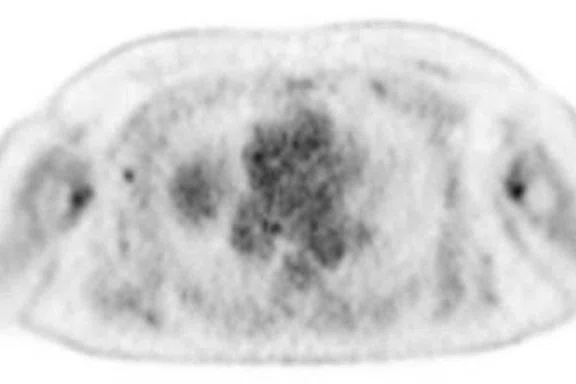
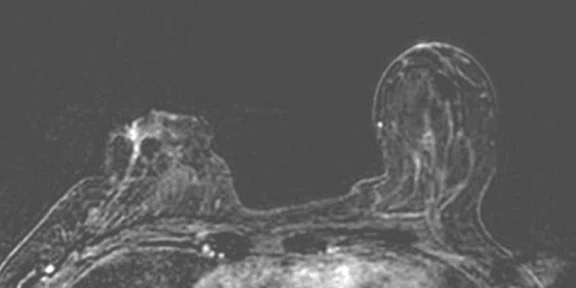
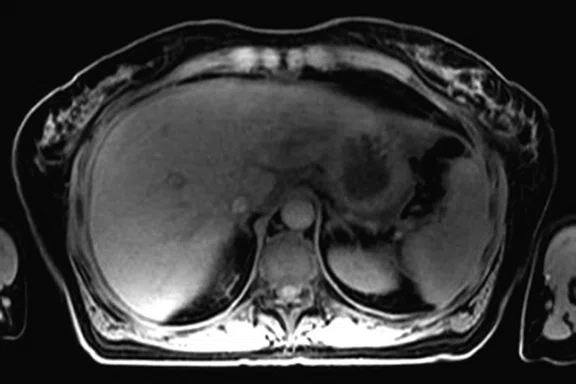
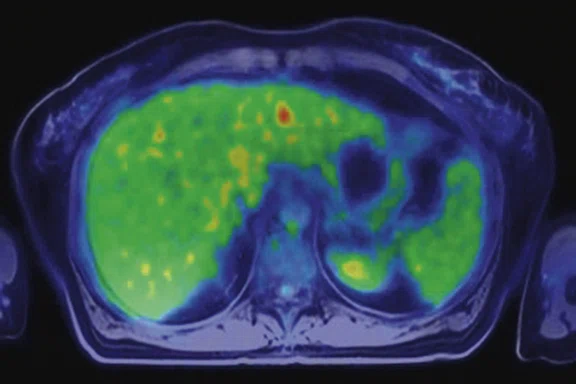
Figure 3.
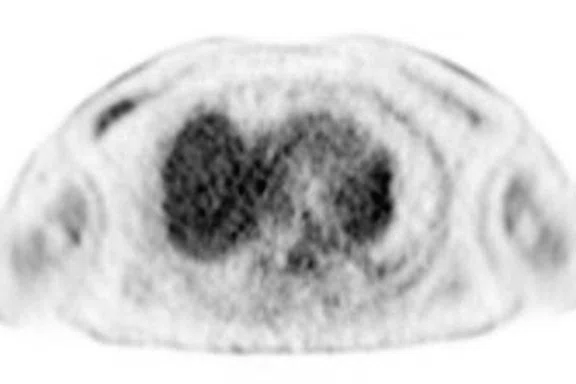
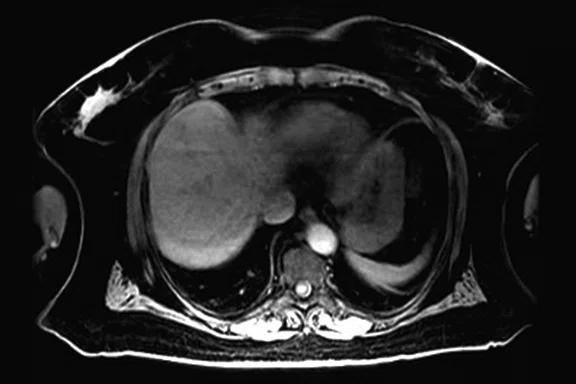
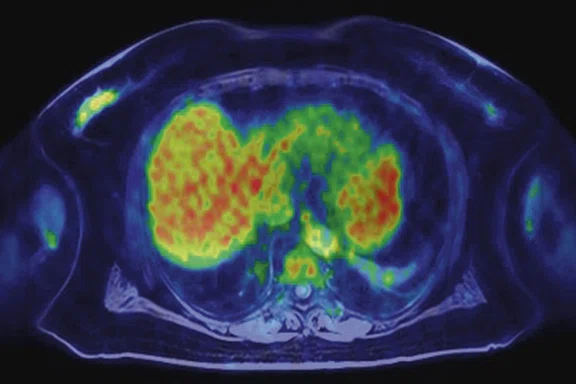
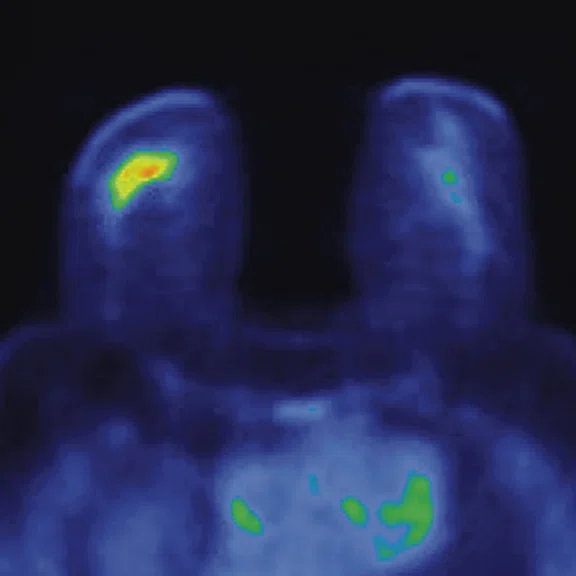
Whole-body supine PET/MR acquisition. (A, D) PET, (B, E) MR and (C, F) fused PET/MR.
B
Figure 3.
Whole-body supine PET/MR acquisition. (A, D) PET, (B, E) MR and (C, F) fused PET/MR.
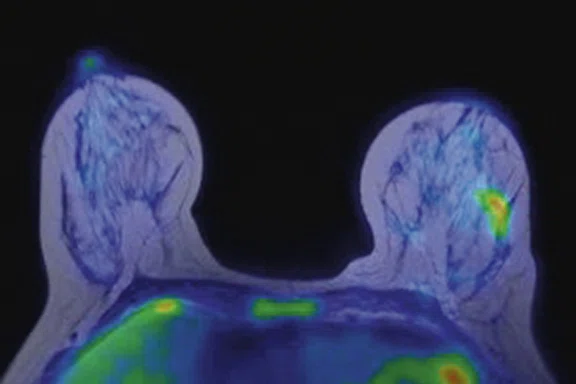
C
Figure 3.
Whole-body supine PET/MR acquisition. (A, D) PET, (B, E) MR and (C, F) fused PET/MR.
D
Figure 3.
Whole-body supine PET/MR acquisition. (A, D) PET, (B, E) MR and (C, F) fused PET/MR.
E
Figure 3.
Whole-body supine PET/MR acquisition. (A, D) PET, (B, E) MR and (C, F) fused PET/MR.
F
Figure 3.
Whole-body supine PET/MR acquisition. (A, D) PET, (B, E) MR and (C, F) fused PET/MR.
A
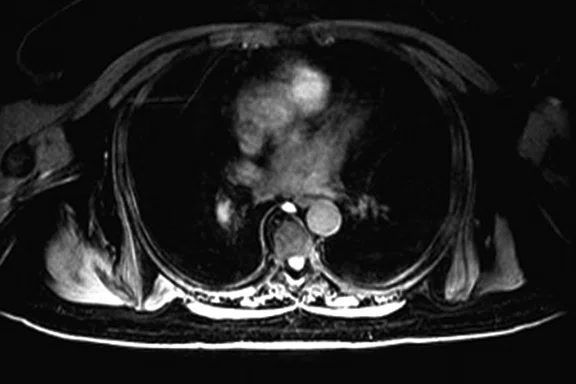
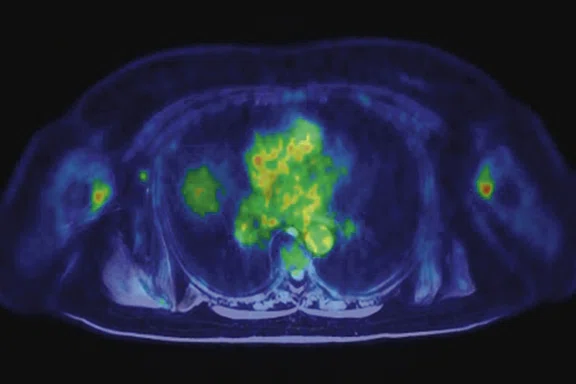
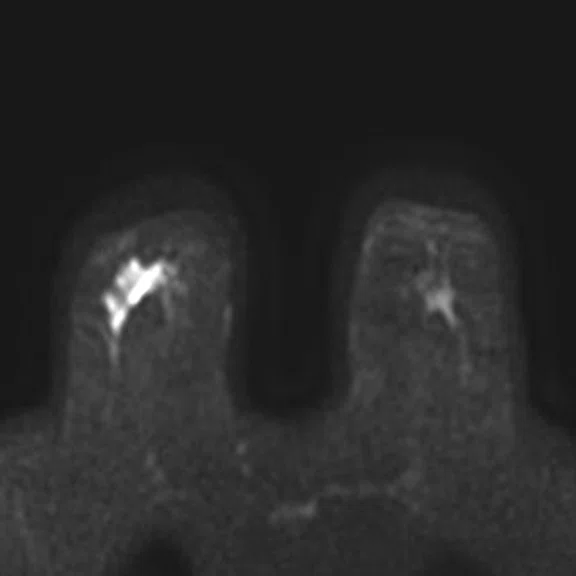
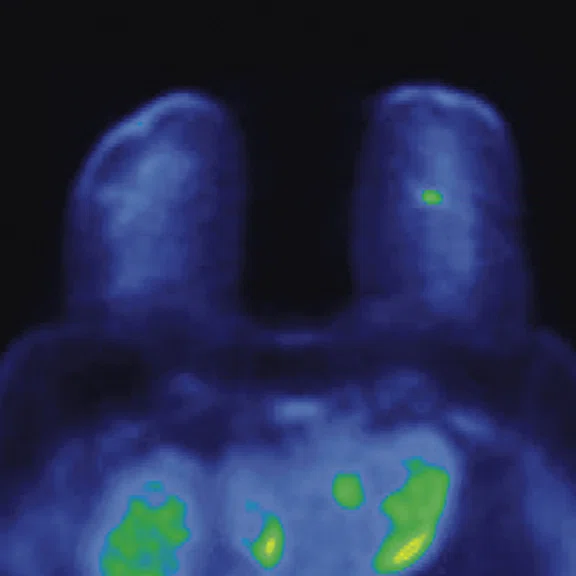
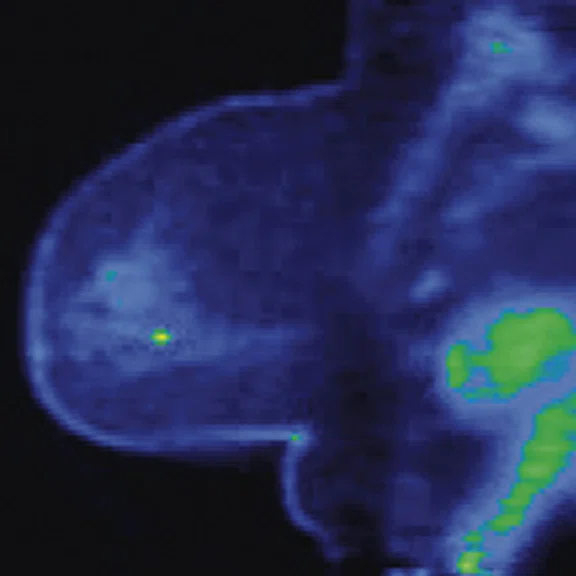
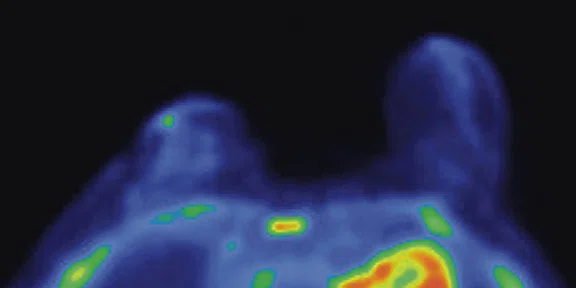
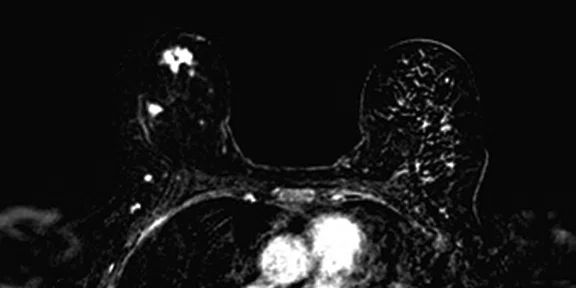
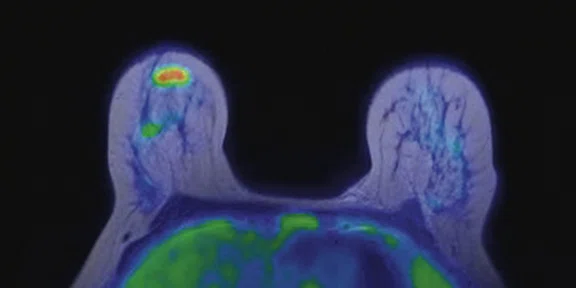
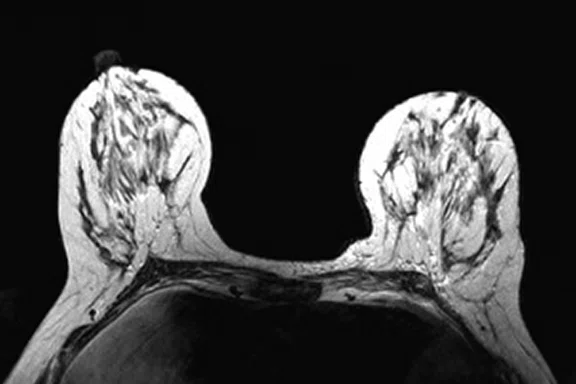
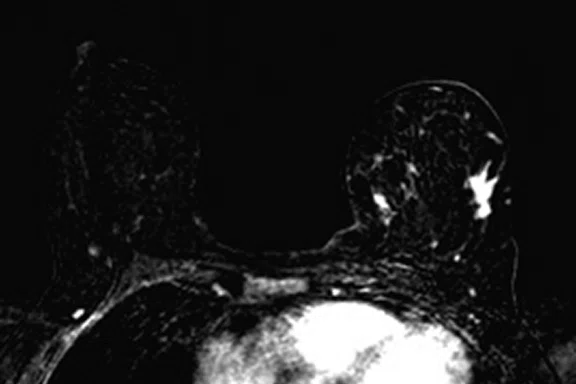
Figure 4.
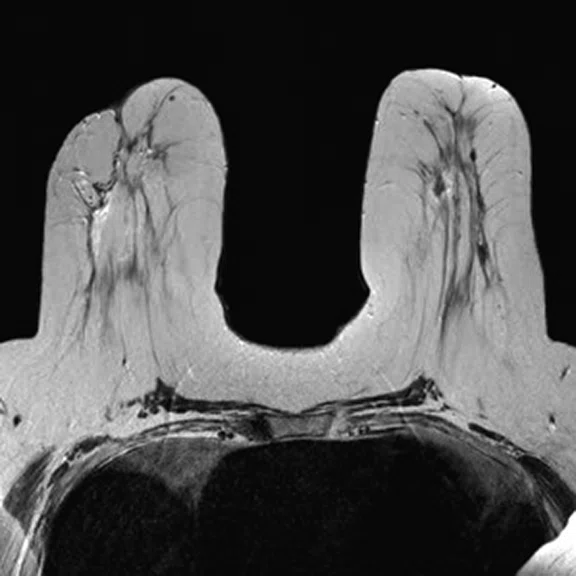
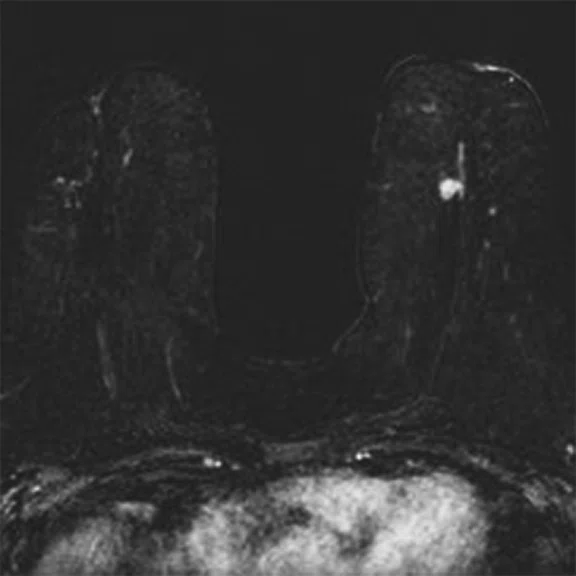
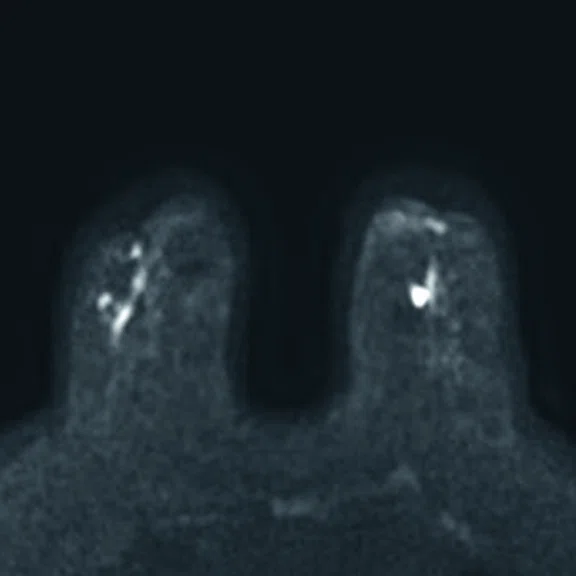
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
B
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
C
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
D
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
E
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
F
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
G
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
1Picchio M, Pampaloni MH. Current status and future perspectives of PET/MRI hybrid imaging. Clin Transl Imaging. 2017;5:79–81 DOI 10.1007/s40336-016-0215-6).
‡11C-methionine is not approved by the US FDA and may not be available for clinical use in all markets.
2Plecha DM, Faulhaber P. PET/MRI of the breast. Eur J Radiol. 2017;94:A26–A34. doi:10.1016/j.ejrad.2017.05.006.
3Chakraborty D, Basu S, Ulaner GA, Alavi A, Kumar R. Diagnostic Role of Fluorodeoxyglucose PET in Breast Cancer: A History to Current Application. PET Clin. 2018;13(3):355–361. doi:10.1016/j.cpet.2018.02.011.
4Lin CY, Lin CL, Kao CH. Staging/restaging performance of F18-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in breast cancer: A review and meta-analysis. Eur J Radiol. 2018;107:158–165. doi:10.1016/j.ejrad.2018.09.003.
5Melsaether AN, Raad RA, Pujara AC, et al. Comparison of Whole-Body (18)F FDG PET/MR Imaging and Whole-Body (18)F FDG PET/CT in Terms of Lesion Detection and Radiation Dose in Patients with Breast Cancer. Radiology. 2016;281(1):193–202. doi:10.1148/radiol.2016151155
6Catalano OA, Nicolai E, Rosen BR, et al. Comparison of CE-FDG-PET/CT with CE-FDG-PET/MR in the evaluation of osseous metastases in breast cancer patients. Br J Cancer. 2015;112(9):1452–1460. doi:10.1038/bjc.2015.112
7Canevari C, Gallivanone F, Zuber V, et al. Prone 18F-FDG PET/CT changes diagnostic and surgical intervention in a breast cancer patient: some considerations about PET/CT imaging acquisition protocol. Clin Imaging. 2015;39(3):506–509. doi:10.1016/j.clinimag.2014.11.005
A
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
B
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
C
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
D
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
E
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
F
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
G
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
H
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
I
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
A
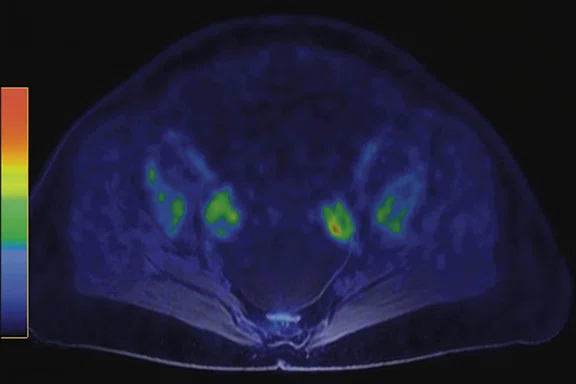
Figure 5.
(A) MR acquisition, (B) fused PET/MR, (C) MR acquisition and (D) PET/MR.
B
Figure 5.
(A) MR acquisition, (B) fused PET/MR, (C) MR acquisition and (D) PET/MR.
C
Figure 5.
(A) MR acquisition, (B) fused PET/MR, (C) MR acquisition and (D) PET/MR.
D
Figure 5.
(A) MR acquisition, (B) fused PET/MR, (C) MR acquisition and (D) PET/MR.
A
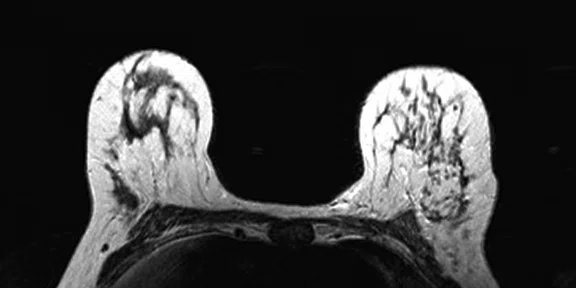
Figure 7.
Prone dedicated acquisition. (A) T2, (B) DWI, (C) post-contrast VIBRANT and (D) fused PET.
B
Figure 7.
Prone dedicated acquisition. (A) T2, (B) DWI, (C) post-contrast VIBRANT and (D) fused PET.
C
Figure 7.
Prone dedicated acquisition. (A) T2, (B) DWI, (C) post-contrast VIBRANT and (D) fused PET.
D
Figure 7.
Prone dedicated acquisition. (A) T2, (B) DWI, (C) post-contrast VIBRANT and (D) fused PET.
A
Figure 8.
Patient follow-up after surgical treatment for breast cancer. Fused PET/MR depicts metastases in the ovaries.
A
Figure 9.
Second PET/MR during chemotherapy for restaging. (A) T2, (B) VIBRANT and (C) fused PET/MR.
B
Figure 9.
Second PET/MR during chemotherapy for restaging. (A) T2, (B) VIBRANT and (C) fused PET/MR.
C
Figure 9.
Second PET/MR during chemotherapy for restaging. (A) T2, (B) VIBRANT and (C) fused PET/MR.
A
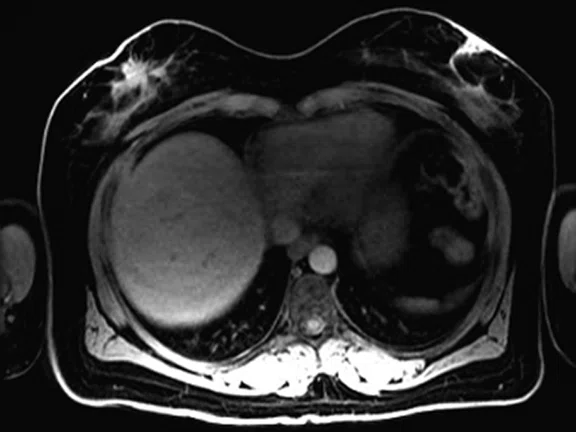
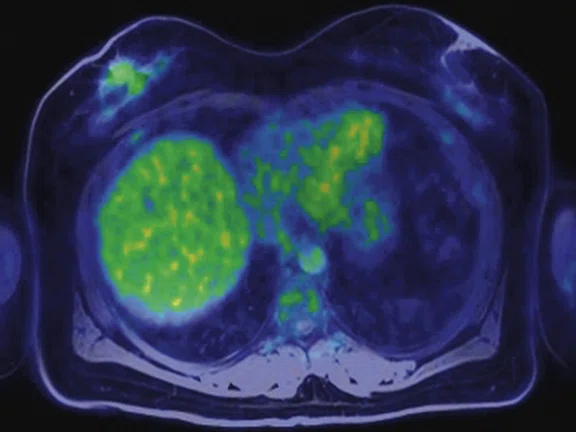
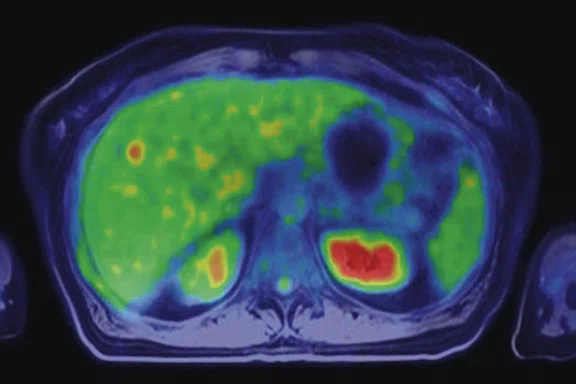
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
B
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
C
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
D
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
E
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
F
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
G
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
8Melsaether A, Moy L. Breast PET/MR Imaging. Radiol Clin North Am. 2017;55(3):579–589. doi:10.1016/j.rcl.2016.12.011.
A
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
B
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
C
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
I
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
D
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
E
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
F
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
G
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
H
Figure 2.
Whole-body supine acquisition. (A) Whole-body PET acquisition, (B) axial PET, (C) MR acquisition, and (D) fused PET/MR. Prone acquisition. (E) Axial T2, (F) post-contrast VIBRANT, (G) axial DWI and (H, I) axial PET/MR.
A
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
B
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
C
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
D
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
E
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
F
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
G
Figure 4.
Prone dedicated acquisition. (A) DWI, (B) axial PET, (C) T2, (D) post-contrast VIBRANT, (E) DWI, (F) axial PET and (G) sagittal PET.
A
Figure 6.
Prone acquisition. (A, C, E) Post-contrast VIBRANT and (B, D, F) PET.
B
Figure 6.
Prone acquisition. (A, C, E) Post-contrast VIBRANT and (B, D, F) PET.
C
Figure 6.
Prone acquisition. (A, C, E) Post-contrast VIBRANT and (B, D, F) PET.
D
Figure 6.
Prone acquisition. (A, C, E) Post-contrast VIBRANT and (B, D, F) PET.
E
Figure 6.
Prone acquisition. (A, C, E) Post-contrast VIBRANT and (B, D, F) PET.
F
Figure 6.
Prone acquisition. (A, C, E) Post-contrast VIBRANT and (B, D, F) PET.
A
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
B
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
C
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
D
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
E
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
F
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
G
Figure 10.
Second PET/MR during chemotherapy demonstrates multiple metastases and infiltrating lobular carcinoma. (A, C) MR acquisition and (B, D) fused PET/MR depicting metastases in the liver. (E) T2, (F) VIBRANT and (G) fused PET/MR showing the lesion in the contralateral breast.
result


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
IN PRACTICE
PET/MR in the evaluation of breast cancer
PET/MR in the evaluation of breast cancer
By Maria Picchio, MD, Associate Professor of Nuclear Medicine at Vita-Salute San Raffaele University and Head of Molecular Imaging Unit, Clinical Research Unit of the Experimental Imaging Centre, Department of Nuclear Medicine, IRCCS San Raffaele Scientific Institute, Milan, Italy
Both PET and MR imaging have an important role in the management of breast cancer, particularly in advanced disease on initial presentation and in therapy monitoring of metastatic disease. MR provides excellent soft tissue characterization for initial diagnosis and to rule out additional malignancies. PET delivers initial staging/restaging and treatment response of patients. When PET is added to MR, it may help improve specificity and reduce false positives. An integrated PET/MR delivers an advantage by providing an evaluation of primary cancer, lymph node involvement and distant metastatic disease in one clinical setting.
Both PET and MR imaging have an important role in the management of breast cancer, particularly in advanced disease on initial presentation and in therapy monitoring of metastatic disease. MR provides excellent soft tissue characterization for initial diagnosis and to rule out additional malignancies. PET delivers initial staging/restaging and treatment response of patients. When PET is added to MR, it may help improve specificity and reduce false positives. An integrated PET/MR delivers an advantage by providing an evaluation of primary cancer, lymph node involvement and distant metastatic disease in one clinical setting.
There are several advantages to using PET/MR for diagnosing cancer in soft tissue, as well as to define the local extent of the disease. PET and MR are often used to provide information on prognosis and treatment response. Therefore, a single integrated PET/MR study with information acquired simultaneously may be particularly beneficial1.
IRCCS San Raffaele Scientific Institute is a university and research hospital established in 1971 to provide specialized care for the most complex and difficult health conditions, with 50,000 inpatients, 1.5 million outpatients, 70,000 emergency services and 30,000 surgical interventions per year. San Raffaele is recognized for the excellence of its teaching and research activities. In 2018, researchers from the campus (University and Scientific Institute) published 1,446 papers, with a mean impact factor of 5.9 and a total impact factor of 8,545. San Raffaele is ranked as one of the leading biomedical research institutes in Italy and Europe.
In early 2018, thanks to a funded grant from the Italian Ministry of Health, our institution installed the first hybrid, fully integrated PET/MR system with Time-of-Flight (TOF) imaging in Italy – the SIGNA™ PET/MR. This new system is in addition to three PET/CT, one SPECT/CT and three SPECT systems in the Nuclear Medicine Unit, headed by Luigi Gianolli, MD. We also have cyclotron, radiochemistry and radiopharmacy labs working under GMP requirements to ensure the quality and safety of radiopharmaceutical production. A pre-clinical facility for small animals is also available. The clinical activity in oncology, neurology and cardiology is complemented by research and methodological programs in our institution.
The first clinical study was performed on the SIGNA™ PET/MR in March 2018. Since then, we have conducted 1,100 exams: 1,014 in oncology, 13 in neurology and 73 in patients with large vessel vasculitis. Our oncology exams by type of radiopharmaceutical tracer are: 720 with 18F-FDG, 162 with 68Ga-DOTATOC, 113 with 11C-choline and 19 with 11C-methionine‡.
The methodological development, set-up, functioning and maintenance of the PET/MR system requires a team that includes PET physicists Valentino Bettinardi, PhD, and Annarita Savi, PhD, and MR expert Paola Scifo, PhD, who together guarantee the optimal functioning, as well as the clinical and research implementation of the PET/MR system. A group of nuclear medicine physicians, Federico Fallanca, MD, Ana Maria Samanes Gajate, MD, Patrizia Magnani, MD, and Carla Canevari, MD, are specifically trained in working with PET/MR and work closely with the radiologists and neuroradiologists. Moreover, Paola Mapelli, MD, a nuclear medicine physician, is currently working toward a PhD with a project focused on the use of PET/MR in prostate cancer and neuroendocrine tumors.
Breast cancer is an area where the combined PET/MR modality delivers an advantage in one clinical setting. In our institution, it is a clinical and clinical research focus with Dr. Canevari as the referring physician for breast cancer studies. PET has an important role in the management of breast cancer, particularly in initial staging, treatment response in metastatic disease and restaging of patients with equivocal findings2-4. MR delivers excellent soft tissue characterization and may be useful to rule out additional malignancy sites. While MR provides high sensitivity but low specificity in the detection of breast cancer, the addition of PET can help improve specificity and reduce false positives. Furthermore, MR is often used to rule out additional malignant sites in Stage 1 cancers that might be mammographically occult5.
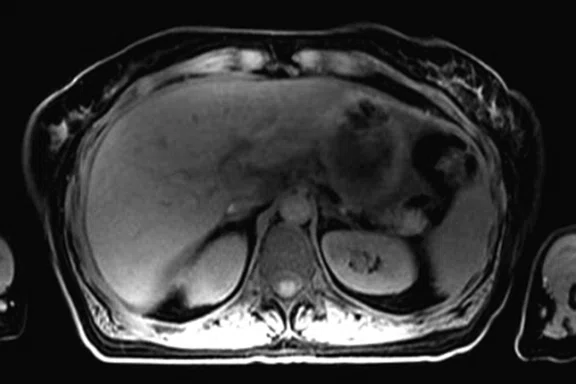
It is also known that MR is better than CT for the detection of metastases in the bone and liver. Catalano, et al, evaluated the staging performance of whole-body DWI, PET/CT and PET/MR in invasive ductal carcinoma of the breast. The authors concluded that when available, PET/MR should be considered for staging these patients because it may also detect bone metastases and very small liver metastases6.
Of the 1,014 PET/MR oncology exams performed in our facility, 178 were breast cancer studies. Sixty-three of these patients had both diagnostic PET and breast MR with contrast. Of these, 44 were for staging and 19 were for evaluation of treatment response or for follow-up of suspected recurrent disease.
Studies have also shown the advantage of imaging breast cancer patients in the prone position7. In our institution, our protocol consists of a whole-body PET/MR acquisition with seven bed positions in the supine position, followed by a high count statistics PET and MR in the prone position using GE’s dedicated 8-channel breast coil.
In less than one hour, we have a complete diagnostic PET and diagnostic MR breast study (Figure 1).
Clinical cases
In the first case, we can visualize the lesion in the left breast (Figure 2A-2D). With the prone breast acquisition, the lesion is well defined and we can also detect a small satellite ipsilateral lesion that was not apparent in the supine position images (Figure 2E-2I).
In another patient with a lesion in the right breast, we can determine the involvement of the axillary lymph node in the supine position (Figure 3). With the acquisition in the prone position, we can more precisely define the anatomical localization of the right lesion (Figure 4A-4B). Furthermore, we could detect another small contralateral lesion that was not seen in the supine whole-body acquisition (Figure 4C-4G).
With the third patient, referred for staging in the right breast (Figure 5), cancer was found to have metastatic involvement. With the high sensitivity PET images obtained during diagnostic MR study in the prone position, we could depict several small bone lesions and axillary lymph node involvement (Figure 6).
The fourth patient was biopsied for infiltrating locular carcinoma (G1) and foci of lobular carcinoma in situ. Lobular carcinoma is a type of breast cancer that has the potential for a false negative in PET. In fact, in this case the PET results were negative. The MR acquisition helped to detect a small lesion not seen in the PET images (Figure 7).
In another patient following surgical treatment of lobular carcinoma, PET/MR was performed to restage the disease. With PET/MR, we could detect bilateral uptake at the ovaries that resulted as metastases from lobular carcinoma after surgical treatment of bilateral salpingo-oophorectomy and omentectomy (Figure 8).
The patient began chemotherapy and underwent a second PET/MR during treatment. The follow-up PET/MR demonstrated the presence of a pathological uptake and a morphological finding in the right breast, which was histologically confirmed to be intraductal carcinoma (Figure 9). Also depicted were multiple metastases in lobular histotype in the liver and the lymph nodes, and another infiltrating lobular carcinoma in the contralateral breast (Figure 10).
This last case underscores a key point that PET/MR can detect very small lesions that may be important for primary cancer and also for the detection of multiple metastatic lesions. This is particularly important in the staging of the disease.
We are currently engaged in several research activities to evaluate the value of PET/MR in breast cancer patients. In particular, one is focused on patients with locally advanced cancer undergoing adjuvant chemotherapy and performing a PET/MR scan before and after treatment. Another clinical trial is using PET/MR for the evaluation of axillary staging in early breast cancer patients who undergo sentinel node biopsy. In addition, our group led by Paola Scifo, PhD, is working to improve attenuation correction maps with optimized MR-based attenuation sequences and examining the effect on PET quantification.
To conclude, breast cancer is an area that we believe PET/MR is useful for evaluating primary cancer, lymph node involvement and distant metastatic disease.
While there are situations where, based on the current knowledge, PET/MR may not provide an advantage compared to PET/CT, such as for local lymph node lesion detection, it appears that PET/MR is overall more sensitive for detecting bone and liver metastases8.
In fact, one specific advantage of a fully integrated PET/MR is the whole-body acquisition where PET, together with MR, may improve the detection of distant metastases.
Moreover, the use of the prone position with dedicated breast MR coils may improve local cancer staging and contrast-enhanced MR may also depict small cancer deposits.
Larger prospective trials are needed to finally understand the breast cancer patient subgroups that will benefit from PET/MR. However, based on our experience, PET/MR may be a one-stop solution for patients with advanced breast cancer and, in particular, in those with a poor prognosis.
In addition, the high performance of the fully integrated PET/MR, specifically with a very high sensitive PET system, an optimal SNR due to the TOF technology and an accurate quantitative reconstruction algorithm makes it a powerful diagnostic tool for clinical and research applications in breast cancer.