‡ Not yet CE marked. Not available for sale in all regions.
result


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
NEWS
SIGNA 7.0T receives US FDA clearance
SIGNA 7.0T receives US FDA clearance
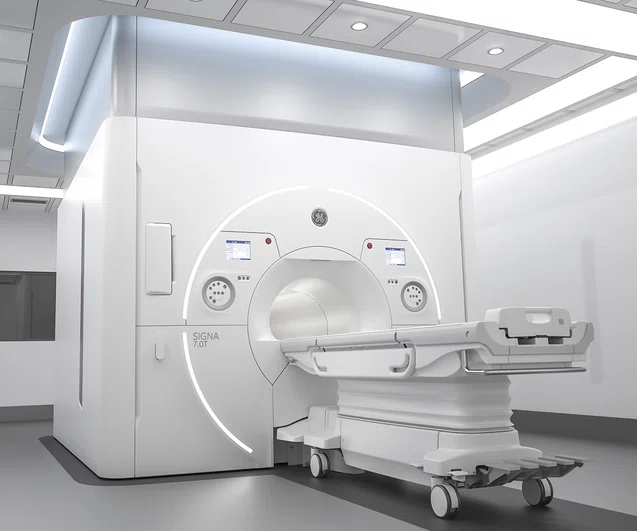
GE Healthcare’s dedication to advancing neurological research and clinical translation with leading institutions around the world continues with the introduction of the newly FDA-cleared SIGNA™ 7.0T‡. The SIGNA™ 7.0T is designed to overcome the limitations of the majority of today’s clinical MR systems by leveraging the ultra-high field magnet technology within its core. With approximately five times higher field strength than most clinical systems, SIGNA™ 7.0T is designed to detect subtle structures that may be significant for clinicians and researchers alike. This new 60-centimeter bore system is designed to be a more powerful tool to image neurodegenerative diseases as well as extremities.
SIGNA™ 7.0T features UltraG gradient technology, GE’s most powerful, whole-body gradient coil, designed to meet the needs of ultra-high field imaging speed and resolution. This system features the familiarity of the SIGNA™Works applications platform so clinicians can use the latest state-of-the-art applications such as deep learning-based platform tools like AIR x™ brain for automated slice positioning and Silent MR imaging. In addition, SIGNA™ 7.0T is equipped with Precision RF transmit and receive architecture designed to enable improved image quality and research flexibility. (See related article Scientific discovery meets clinical translation at 7.0T.)








