‡ SIGNA™ Champion is 510(k) cleared with the US FDA. Not CE marked. Not available in all regions.
1. https://data.oecd.org/healtheqt/magnetic-resonance-imaging-mri-units.
result


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
News
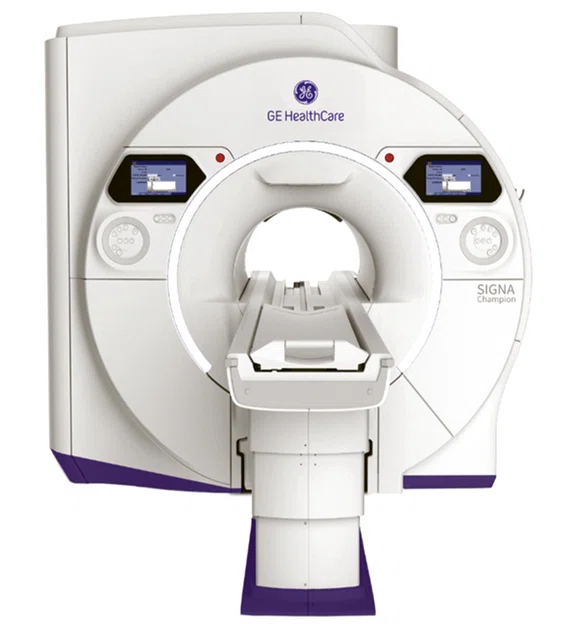
SIGNA™ Champion nets US FDA clearance
SIGNA™ Champion nets US FDA clearance
SIGNA™ Champion‡, a 1.5T MR system designed to enhance the standard care of MR exams for patients everywhere by democratizing advanced AI and innovative features to help enable faster and more precise MRI scans, has received US FDA clearance.
60 to 70% of the world population lacks access to MRI.1 While more patients gain access to MRI technology every year, they undergo a wide variation of quality, comfort & overall experience from their scans. Enabling equity in both the quality and experience of every MRI exam is equally as important as improving access to MRI for patients around the world.
In the pursuit of healthcare access and equity, SIGNA Champion is designed to stand out as an exceptional high-performance 1.5T wide bore MRI system powered by a variety of GE HealthCare innovative breakthroughs technologies, including AIR™ Recon DL, Sonic DL™ and AIR™ Coils.
SIGNA™ Champion is GE HealthCare’s smallest footprint and most power efficient 1.5T wide bore system. With shorter scans times, the system is designed to save power, increase throughput, and enhance the overall patient experience. With its highly scalable platform, SIGNA™ Champion is designed to support broader affordability, configurability, and upgradability to support services expansion for health systems. This can help improve health equity, allowing a wider range of access to the most advanced technologies for all patients.
DOWNLOAD ARTICLE HERE