result
A
Figure 1.
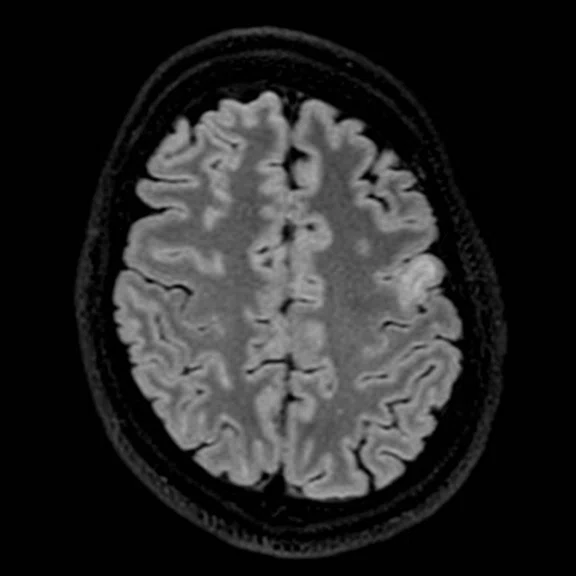
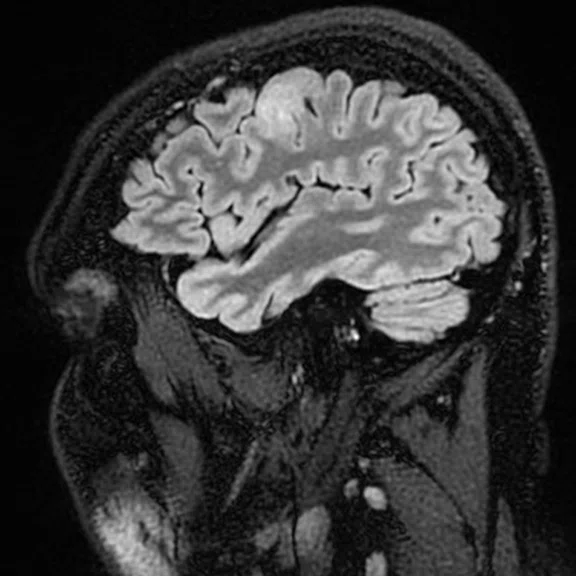
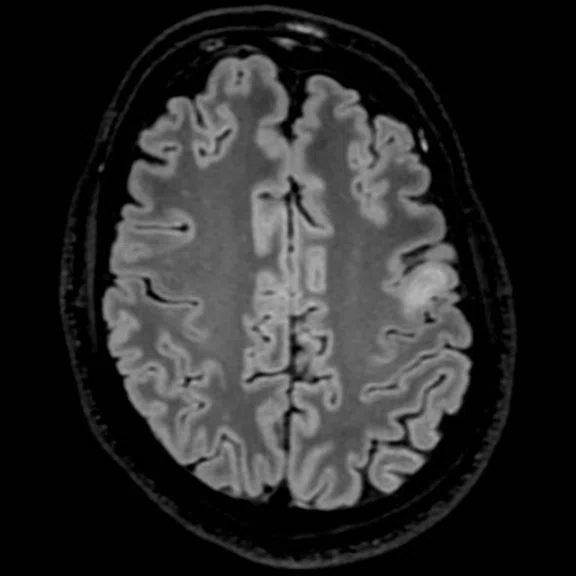
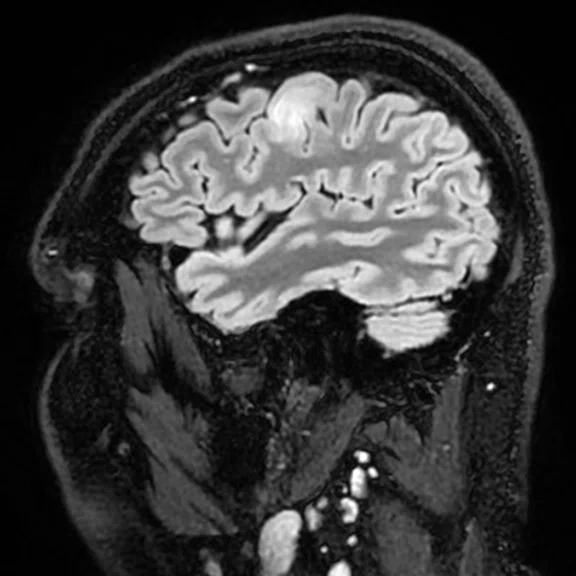
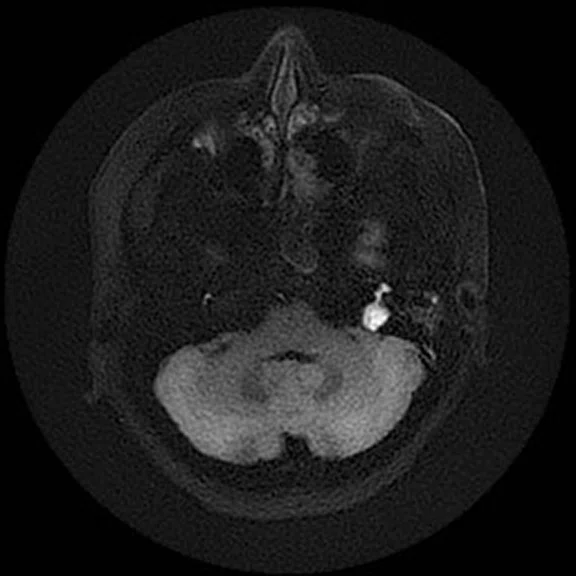
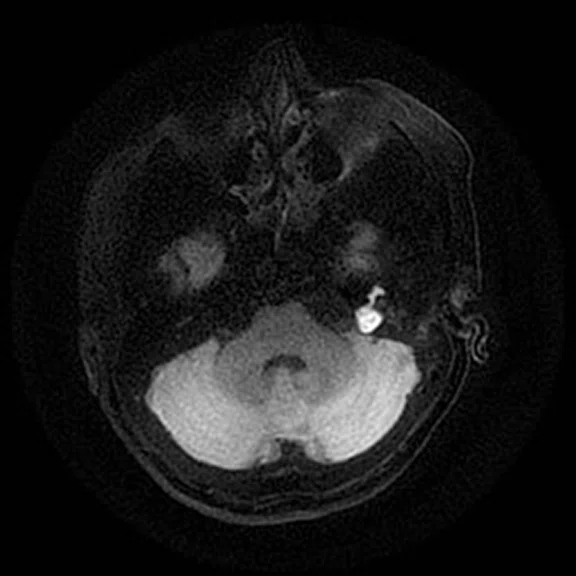
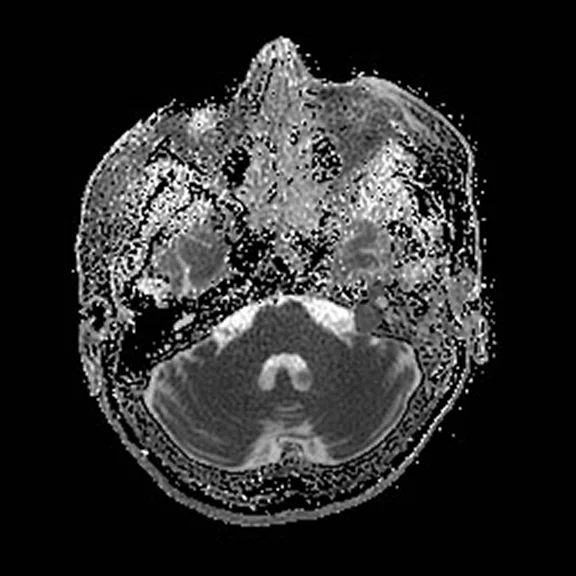
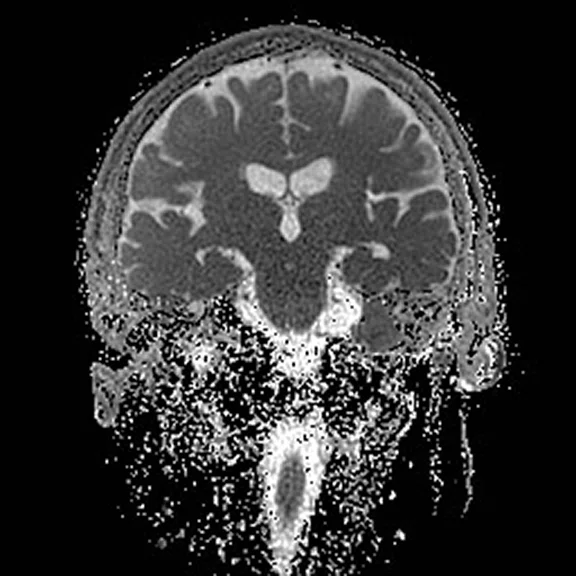
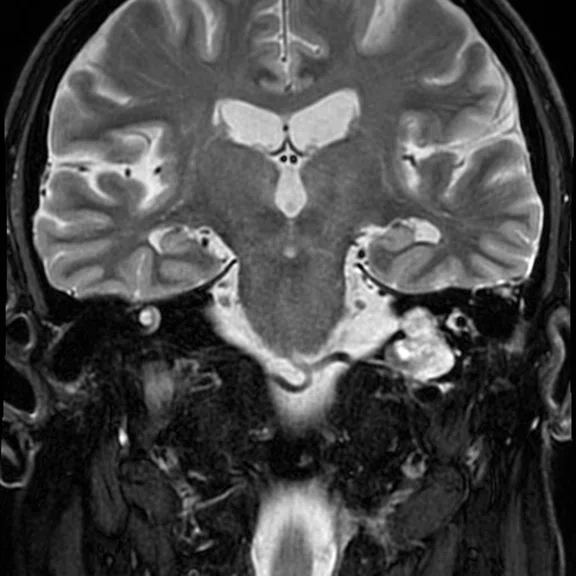
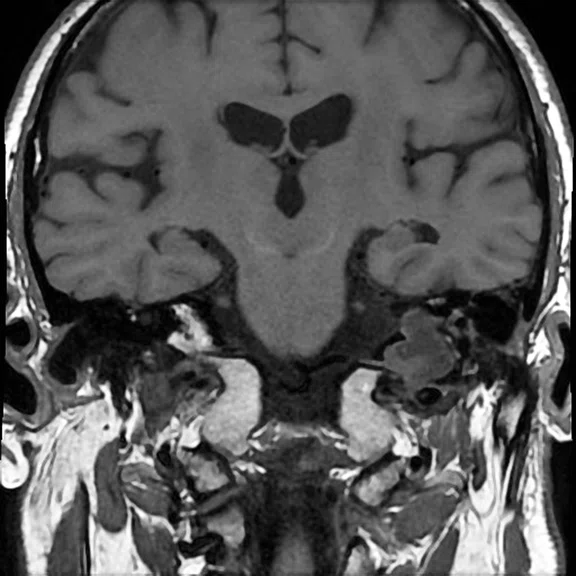
With the upgrade to SIGNA™ Premier, T2 FLAIR Cube FatSat sequences provide even greater definition of cortical anatomy and gray/white matter interfaces on thin slice 3D datasets, which improves lesion conspicuity and delineation. (A-B) Discovery™ MR750 with SIGNA™Works (DV26) and 8ch head coil; (C-D) SIGNA™ Premier with SIGNA™Works AIR™ Edition (DV28) and AIR™ 48ch Head Coil.
B
Figure 1.
With the upgrade to SIGNA™ Premier, T2 FLAIR Cube FatSat sequences provide even greater definition of cortical anatomy and gray/white matter interfaces on thin slice 3D datasets, which improves lesion conspicuity and delineation. (A-B) Discovery™ MR750 with SIGNA™Works (DV26) and 8ch head coil; (C-D) SIGNA™ Premier with SIGNA™Works AIR™ Edition (DV28) and AIR™ 48ch Head Coil.
C
Figure 1.
With the upgrade to SIGNA™ Premier, T2 FLAIR Cube FatSat sequences provide even greater definition of cortical anatomy and gray/white matter interfaces on thin slice 3D datasets, which improves lesion conspicuity and delineation. (A-B) Discovery™ MR750 with SIGNA™Works (DV26) and 8ch head coil; (C-D) SIGNA™ Premier with SIGNA™Works AIR™ Edition (DV28) and AIR™ 48ch Head Coil.
D
Figure 1.
With the upgrade to SIGNA™ Premier, T2 FLAIR Cube FatSat sequences provide even greater definition of cortical anatomy and gray/white matter interfaces on thin slice 3D datasets, which improves lesion conspicuity and delineation. (A-B) Discovery™ MR750 with SIGNA™Works (DV26) and 8ch head coil; (C-D) SIGNA™ Premier with SIGNA™Works AIR™ Edition (DV28) and AIR™ 48ch Head Coil.
A
Figure 2.
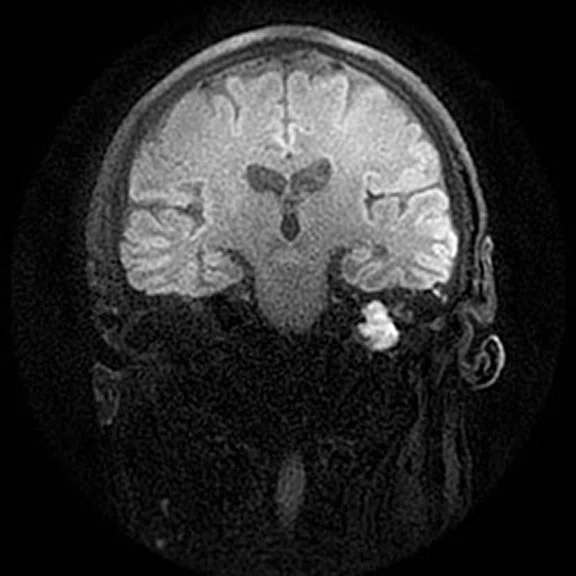
Using T1 Cube FatSat post contrast, we can make accurate co-registered comparisons between interval studies. This increases our sensitivity and confidence for identifying early changes in cases of complex skull base disease and perineural extension. This sequence is also of great utility for fusing with radiotherapy planning scans. With the SIGNA™ Premier 3.0T system, we are achieving even better results than previously. (A-C) Comparison images (right + left, top + bottom) demonstrate recurrence and interval progression of residual and previously treated adenoid cystic carcinoma. There is extension along the infraorbital nerve, within the pterygopalatine fossa and along the maxillary branch of the trigeminal nerve via foramen rotundum. The left and top of the comparison images are obtained on the SIGNA™ Premier 3.0T, compared to the Optima™ MR450 1.5T system (right and bottom). The images show how clearly the infraorbital nerve disease is demonstrated at 3.0T, and how this region is more clearly demonstrated than on the older 1.5T scanner.
B
Figure 2.
Using T1 Cube FatSat post contrast, we can make accurate co-registered comparisons between interval studies. This increases our sensitivity and confidence for identifying early changes in cases of complex skull base disease and perineural extension. This sequence is also of great utility for fusing with radiotherapy planning scans. With the SIGNA™ Premier 3.0T system, we are achieving even better results than previously. (A-C) Comparison images (right + left, top + bottom) demonstrate recurrence and interval progression of residual and previously treated adenoid cystic carcinoma. There is extension along the infraorbital nerve, within the pterygopalatine fossa and along the maxillary branch of the trigeminal nerve via foramen rotundum. The left and top of the comparison images are obtained on the SIGNA™ Premier 3.0T, compared to the Optima™ MR450 1.5T system (right and bottom). The images show how clearly the infraorbital nerve disease is demonstrated at 3.0T, and how this region is more clearly demonstrated than on the older 1.5T scanner.
C
Figure 2.
Using T1 Cube FatSat post contrast, we can make accurate co-registered comparisons between interval studies. This increases our sensitivity and confidence for identifying early changes in cases of complex skull base disease and perineural extension. This sequence is also of great utility for fusing with radiotherapy planning scans. With the SIGNA™ Premier 3.0T system, we are achieving even better results than previously. (A-C) Comparison images (right + left, top + bottom) demonstrate recurrence and interval progression of residual and previously treated adenoid cystic carcinoma. There is extension along the infraorbital nerve, within the pterygopalatine fossa and along the maxillary branch of the trigeminal nerve via foramen rotundum. The left and top of the comparison images are obtained on the SIGNA™ Premier 3.0T, compared to the Optima™ MR450 1.5T system (right and bottom). The images show how clearly the infraorbital nerve disease is demonstrated at 3.0T, and how this region is more clearly demonstrated than on the older 1.5T scanner.
A
Figure 3.
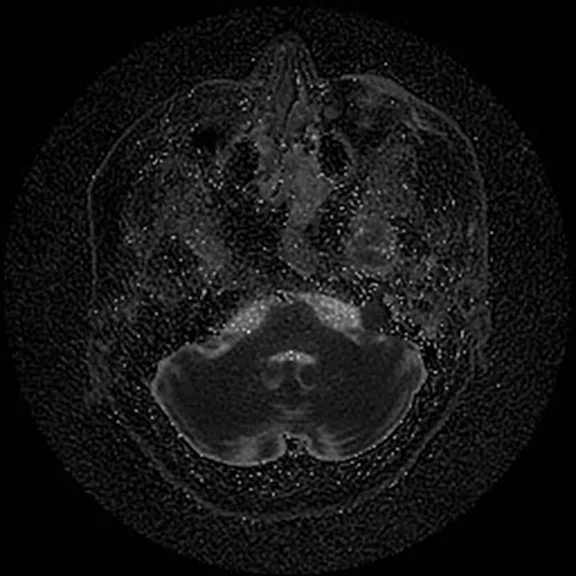
In this case, the left frontal lobe glioma is clearly demonstrated on the (A) T2 FLAIR sequence and (B) post-contrast 3D BRAVO. (D) The ASL allows identification of a well demarcated region of higher cerebral blood flow concerning for a focus of higher grade disease/transformation. In our experience we see changes on ASL more clearly than we do on (C) DSC perfusion imaging-rCBV in cases of non-operated gliomas beginning to undergo transformation. Cross referenced to the other sequences, this assists with the treatment planning. (E) The DTI color maps are helpful in suggesting the possibility of a degree of posterior displacement of at least some of the descending motor tracts which assists in surgical planning and informed consent. (F) Multivoxel spectroscopy allows generation of choline/NAA maps which can also be helpful in demonstrating tumour heterogeneity and the likely extent of tumor. This patient underwent awake craniotomy and near total resection.
B
Figure 3.
In this case, the left frontal lobe glioma is clearly demonstrated on the (A) T2 FLAIR sequence and (B) post-contrast 3D BRAVO. (D) The ASL allows identification of a well demarcated region of higher cerebral blood flow concerning for a focus of higher grade disease/transformation. In our experience we see changes on ASL more clearly than we do on (C) DSC perfusion imaging-rCBV in cases of non-operated gliomas beginning to undergo transformation. Cross referenced to the other sequences, this assists with the treatment planning. (E) The DTI color maps are helpful in suggesting the possibility of a degree of posterior displacement of at least some of the descending motor tracts which assists in surgical planning and informed consent. (F) Multivoxel spectroscopy allows generation of choline/NAA maps which can also be helpful in demonstrating tumour heterogeneity and the likely extent of tumor. This patient underwent awake craniotomy and near total resection.
C
Figure 3.
In this case, the left frontal lobe glioma is clearly demonstrated on the (A) T2 FLAIR sequence and (B) post-contrast 3D BRAVO. (D) The ASL allows identification of a well demarcated region of higher cerebral blood flow concerning for a focus of higher grade disease/transformation. In our experience we see changes on ASL more clearly than we do on (C) DSC perfusion imaging-rCBV in cases of non-operated gliomas beginning to undergo transformation. Cross referenced to the other sequences, this assists with the treatment planning. (E) The DTI color maps are helpful in suggesting the possibility of a degree of posterior displacement of at least some of the descending motor tracts which assists in surgical planning and informed consent. (F) Multivoxel spectroscopy allows generation of choline/NAA maps which can also be helpful in demonstrating tumour heterogeneity and the likely extent of tumor. This patient underwent awake craniotomy and near total resection.
D
Figure 3.
In this case, the left frontal lobe glioma is clearly demonstrated on the (A) T2 FLAIR sequence and (B) post-contrast 3D BRAVO. (D) The ASL allows identification of a well demarcated region of higher cerebral blood flow concerning for a focus of higher grade disease/transformation. In our experience we see changes on ASL more clearly than we do on (C) DSC perfusion imaging-rCBV in cases of non-operated gliomas beginning to undergo transformation. Cross referenced to the other sequences, this assists with the treatment planning. (E) The DTI color maps are helpful in suggesting the possibility of a degree of posterior displacement of at least some of the descending motor tracts which assists in surgical planning and informed consent. (F) Multivoxel spectroscopy allows generation of choline/NAA maps which can also be helpful in demonstrating tumour heterogeneity and the likely extent of tumor. This patient underwent awake craniotomy and near total resection.
E
Figure 3.
In this case, the left frontal lobe glioma is clearly demonstrated on the (A) T2 FLAIR sequence and (B) post-contrast 3D BRAVO. (D) The ASL allows identification of a well demarcated region of higher cerebral blood flow concerning for a focus of higher grade disease/transformation. In our experience we see changes on ASL more clearly than we do on (C) DSC perfusion imaging-rCBV in cases of non-operated gliomas beginning to undergo transformation. Cross referenced to the other sequences, this assists with the treatment planning. (E) The DTI color maps are helpful in suggesting the possibility of a degree of posterior displacement of at least some of the descending motor tracts which assists in surgical planning and informed consent. (F) Multivoxel spectroscopy allows generation of choline/NAA maps which can also be helpful in demonstrating tumour heterogeneity and the likely extent of tumor. This patient underwent awake craniotomy and near total resection.
F
Figure 3.
In this case, the left frontal lobe glioma is clearly demonstrated on the (A) T2 FLAIR sequence and (B) post-contrast 3D BRAVO. (D) The ASL allows identification of a well demarcated region of higher cerebral blood flow concerning for a focus of higher grade disease/transformation. In our experience we see changes on ASL more clearly than we do on (C) DSC perfusion imaging-rCBV in cases of non-operated gliomas beginning to undergo transformation. Cross referenced to the other sequences, this assists with the treatment planning. (E) The DTI color maps are helpful in suggesting the possibility of a degree of posterior displacement of at least some of the descending motor tracts which assists in surgical planning and informed consent. (F) Multivoxel spectroscopy allows generation of choline/NAA maps which can also be helpful in demonstrating tumour heterogeneity and the likely extent of tumor. This patient underwent awake craniotomy and near total resection.
A
Figure 4.
The PROPELLER Duo sequence allows for axial and coronal DWI acquisitions with automatic generation of ADC maps. This is demonstrated nicely in this patient with a skull base epidermoid. Such detail helps assist in the definition of the anatomical extent of epidermoids/cholesteatomas, particularly when there are also inflammatory changes within the mastoid air cells and middle ear. (A, D) Discovery™ MR750 with SIGNA™Works (DV26) and 8ch head coil; (B, C, E, F ) SIGNA™ Premier with SIGNA™Works AIR™ Edition (DV28) and AIR™ 48ch Head Coil.
B
Figure 4.
The PROPELLER Duo sequence allows for axial and coronal DWI acquisitions with automatic generation of ADC maps. This is demonstrated nicely in this patient with a skull base epidermoid. Such detail helps assist in the definition of the anatomical extent of epidermoids/cholesteatomas, particularly when there are also inflammatory changes within the mastoid air cells and middle ear. (A, D) Discovery™ MR750 with SIGNA™Works (DV26) and 8ch head coil; (B, C, E, F ) SIGNA™ Premier with SIGNA™Works AIR™ Edition (DV28) and AIR™ 48ch Head Coil.
C
Figure 4.
The PROPELLER Duo sequence allows for axial and coronal DWI acquisitions with automatic generation of ADC maps. This is demonstrated nicely in this patient with a skull base epidermoid. Such detail helps assist in the definition of the anatomical extent of epidermoids/cholesteatomas, particularly when there are also inflammatory changes within the mastoid air cells and middle ear. (A, D) Discovery™ MR750 with SIGNA™Works (DV26) and 8ch head coil; (B, C, E, F ) SIGNA™ Premier with SIGNA™Works AIR™ Edition (DV28) and AIR™ 48ch Head Coil.
D
Figure 4.
The PROPELLER Duo sequence allows for axial and coronal DWI acquisitions with automatic generation of ADC maps. This is demonstrated nicely in this patient with a skull base epidermoid. Such detail helps assist in the definition of the anatomical extent of epidermoids/cholesteatomas, particularly when there are also inflammatory changes within the mastoid air cells and middle ear. (A, D) Discovery™ MR750 with SIGNA™Works (DV26) and 8ch head coil; (B, C, E, F ) SIGNA™ Premier with SIGNA™Works AIR™ Edition (DV28) and AIR™ 48ch Head Coil.
E
Figure 4.
The PROPELLER Duo sequence allows for axial and coronal DWI acquisitions with automatic generation of ADC maps. This is demonstrated nicely in this patient with a skull base epidermoid. Such detail helps assist in the definition of the anatomical extent of epidermoids/cholesteatomas, particularly when there are also inflammatory changes within the mastoid air cells and middle ear. (A, D) Discovery™ MR750 with SIGNA™Works (DV26) and 8ch head coil; (B, C, E, F ) SIGNA™ Premier with SIGNA™Works AIR™ Edition (DV28) and AIR™ 48ch Head Coil.
F
Figure 4.
The PROPELLER Duo sequence allows for axial and coronal DWI acquisitions with automatic generation of ADC maps. This is demonstrated nicely in this patient with a skull base epidermoid. Such detail helps assist in the definition of the anatomical extent of epidermoids/cholesteatomas, particularly when there are also inflammatory changes within the mastoid air cells and middle ear. (A, D) Discovery™ MR750 with SIGNA™Works (DV26) and 8ch head coil; (B, C, E, F ) SIGNA™ Premier with SIGNA™Works AIR™ Edition (DV28) and AIR™ 48ch Head Coil.
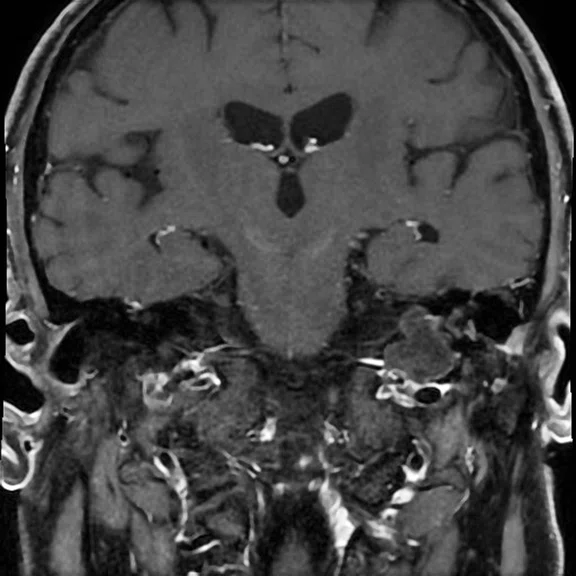
A
Figure 5.
Same patient as Figure 4. Note the skull base epidermoid demonstrated on (A) STIR Cube, (B) T1 Cube and (C) post-contrast T1 Cube FatSat sequences. These are robust sequences which, together, can provide accurate delineation of skull base lesions while also being able to fuse images to radiotherapy planning data sets. The clear delineation of flow voids on T1 Cube and cross reference to the PROPELLER Duo sequence can also be useful in assisting with surgical planning in cases of cholesteatoma, if there is a dehiscent internal carotid artery and cholesteatoma extent is not clearly defined on CT due to additional inflammatory changes.
B
Figure 5.
Same patient as Figure 4. Note the skull base epidermoid demonstrated on (A) STIR Cube, (B) T1 Cube and (C) post-contrast T1 Cube FatSat sequences. These are robust sequences which, together, can provide accurate delineation of skull base lesions while also being able to fuse images to radiotherapy planning data sets. The clear delineation of flow voids on T1 Cube and cross reference to the PROPELLER Duo sequence can also be useful in assisting with surgical planning in cases of cholesteatoma, if there is a dehiscent internal carotid artery and cholesteatoma extent is not clearly defined on CT due to additional inflammatory changes.
C
Figure 5.
Same patient as Figure 4. Note the skull base epidermoid demonstrated on (A) STIR Cube, (B) T1 Cube and (C) post-contrast T1 Cube FatSat sequences. These are robust sequences which, together, can provide accurate delineation of skull base lesions while also being able to fuse images to radiotherapy planning data sets. The clear delineation of flow voids on T1 Cube and cross reference to the PROPELLER Duo sequence can also be useful in assisting with surgical planning in cases of cholesteatoma, if there is a dehiscent internal carotid artery and cholesteatoma extent is not clearly defined on CT due to additional inflammatory changes.
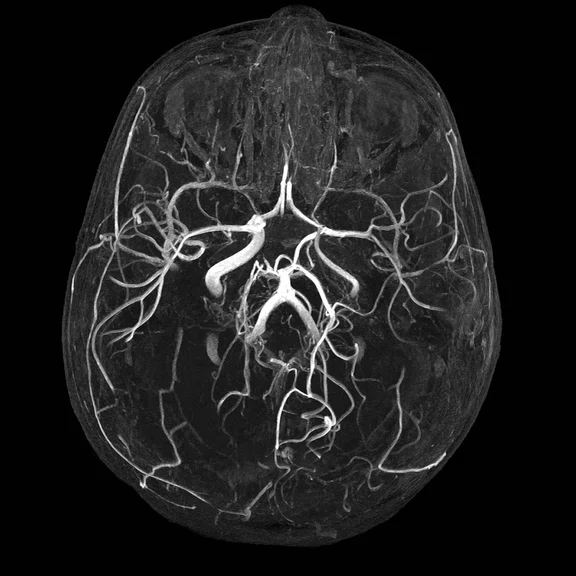
A
Figure 6.
This 3-slab Time of Flight (TOF) dataset was acquired from a pediatric patient without sedation or anesthesia. The sparsity of TOF imaging and increased SNR from the AIR™ 48ch Head Coil favor higher compressed sensing factors. The team are routinely able to acquire 720 x 512 matrix sizes using ARC=2 and HyperSense=1.8 in clinically viable times and excellent resolution with a 0.3 x 0.4 x 0.3 mm voxel size. Hull also utilizes FatSat and Magnetization Transfer options on their protocol to further enhance vessel conspicuity.
A
Figure 7.
Sequences such as (A) 3D FLAIR Cube axial reformat, (D) post-contrast 3D BRAVO and (E) T2 PROPELLER are complemented by the breadth of functional imaging sequences available on the SIGNA™ Premier 3.0T system. In this case (F) CBF derived from 3D PCASL, (B) rCBV calculated from T2* DSC and (C) choline/NAA 3D multivoxel spectroscopy demonstrate high grade features in a region of tumor without gross pathological enhancement.
B
Figure 7.
Sequences such as (A) 3D FLAIR Cube axial reformat, (D) post-contrast 3D BRAVO and (E) T2 PROPELLER are complemented by the breadth of functional imaging sequences available on the SIGNA™ Premier 3.0T system. In this case (F) CBF derived from 3D PCASL, (B) rCBV calculated from T2* DSC and (C) choline/NAA 3D multivoxel spectroscopy demonstrate high grade features in a region of tumor without gross pathological enhancement.
C
Figure 7.
Sequences such as (A) 3D FLAIR Cube axial reformat, (D) post-contrast 3D BRAVO and (E) T2 PROPELLER are complemented by the breadth of functional imaging sequences available on the SIGNA™ Premier 3.0T system. In this case (F) CBF derived from 3D PCASL, (B) rCBV calculated from T2* DSC and (C) choline/NAA 3D multivoxel spectroscopy demonstrate high grade features in a region of tumor without gross pathological enhancement.
D
Figure 7.
Sequences such as (A) 3D FLAIR Cube axial reformat, (D) post-contrast 3D BRAVO and (E) T2 PROPELLER are complemented by the breadth of functional imaging sequences available on the SIGNA™ Premier 3.0T system. In this case (F) CBF derived from 3D PCASL, (B) rCBV calculated from T2* DSC and (C) choline/NAA 3D multivoxel spectroscopy demonstrate high grade features in a region of tumor without gross pathological enhancement.
E
Figure 7.
Sequences such as (A) 3D FLAIR Cube axial reformat, (D) post-contrast 3D BRAVO and (E) T2 PROPELLER are complemented by the breadth of functional imaging sequences available on the SIGNA™ Premier 3.0T system. In this case (F) CBF derived from 3D PCASL, (B) rCBV calculated from T2* DSC and (C) choline/NAA 3D multivoxel spectroscopy demonstrate high grade features in a region of tumor without gross pathological enhancement.
F
Figure 7.
Sequences such as (A) 3D FLAIR Cube axial reformat, (D) post-contrast 3D BRAVO and (E) T2 PROPELLER are complemented by the breadth of functional imaging sequences available on the SIGNA™ Premier 3.0T system. In this case (F) CBF derived from 3D PCASL, (B) rCBV calculated from T2* DSC and (C) choline/NAA 3D multivoxel spectroscopy demonstrate high grade features in a region of tumor without gross pathological enhancement.


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
IN PRACTICE
Advanced neuroimaging at Hull gets a big lift
Advanced neuroimaging at Hull gets a big lift
by Lawrence Kenning, PhD, Medical Physicist, Martin Pickles, PhD, MRI Section Manager, and Richard List, FRCR, EDiNR, Consultant Radiologist in Head and Neck and Neuroradiology, Hull University Teaching Hospitals NHS Trust, Hull, United Kingdom
In August 2020, Hull University Teaching Hospitals NHS Trust upgraded a Discovery™ MR750 3.0T with a 60 cm bore to a SIGNA™ Premier 3.0T with a 70 cm bore via the SIGNA™ Lift program. The increase in bore size allows us to accommodate more patients in our 3.0T scanner, particularly those with a larger body habitus and patients that are anxious or claustrophobic.
In August 2020, Hull University Teaching Hospitals NHS Trust upgraded a Discovery™ MR750 3.0T with a 60 cm bore to a SIGNA™ Premier 3.0T with a 70 cm bore via the SIGNA™ Lift program. The increase in bore size allows us to accommodate more patients in our 3.0T scanner, particularly those with a larger body habitus and patients that are anxious or claustrophobic.
Making the leap from 60 cm to 70 cm has traditionally led to a compromise in gradient performance. As a tertiary institution, where complex and difficult cases are referred from other hospitals, it was imperative to maintain the same high-quality imaging as the 60 cm bore Discovery™ MR750. Due to hardware and software improvements, the larger- bore SIGNA™ Premier can maintain or even surpass the image quality of the Discovery™ MR750.
Hardware improvements
An important differentiator of the SIGNA™ Premier is the SuperG gradients, enabling 80 mT/m and 200 T/m/s compared to 50 mT/m and 200 T/m/s on Discovery™ MR750. The ability to achieve the gradient performance of a 60 cm system on a 70 cm bore scanner has thus far been remarkable, and we have vastly improved the patient experience.
The increase in total RF channels from 32 on the Discovery™ MR750 to 146 on the SIGNA™ Premier has enabled a huge leap forward in terms of coils, SNR and acceleration factor. This has allowed us to go from an 8-channel head coil to the AIR™ 48-channel Head Coil. We also utilize a 16-channel cervical spine coil, the 30-channel AIR™ Anterior Array (AA) Coil and the 60-channel AIR™ Posterior Array (PA) Coil for routine imaging.
With 48 channels in the head coil, we can achieve higher SNR for neuro sequences such as MR spectroscopy (MRS), 3D Arterial Spin Labeling (ASL) and FLAIR Cube. The AIR™ 48ch Head Coil design delivers a better g-factor, and the coil circuitry is closer together without negatively impacting SNR. Together, these two design innovations enable the use of higher levels of acceleration/parallel imaging without incurring artifacts that degrade image quality. Additionally, the AIR™ 48ch Head Coil has more coil elements in the z-direction, making it amenable to utilizing HyperBand in diffusion- weighted imaging (DWI), diffusion tensor imaging (DTI) and dynamic susceptibility contrast-enhanced (DSC) sequences.
Plus, the AIR™ 48ch Head Coil is compatible with Comfort Tilt, a device that improves patient comfort and accommodates kyphotic patients. We’ve found that during longer, more detailed neuro studies, the patient is more compliant because they are more comfortable. A spacer in the coil increases the A/P size for use in patients with larger heads and necks, extending the technological gains to a greater patient cohort.
By taking advantage of the higher RF channels and coil design, we’ve increased ARC in both phase and slice directions on FLAIR Cube from 2x2 to 3x2 – moving from a four- to six-fold increase in acceleration. At Hull, our priority is image quality, so while our scan times are slightly shorter – our FLAIR Cube sequence takes 4:57 minutes and is 40 seconds shorter on SIGNA™ Premier than the prior scanner – our image quality has improved drastically. For other institutions more focused on shortening scan times, the SNR gain can be utilized to achieve that goal. With the higher acceleration capabilities, we’ve shortened the echo train length (ETL), resulting in reduced image blurring, which also contributes to higher overall image quality. As with FLAIR Cube, we’ve been able to increase ARC acceleration to 3x2 for T2 Cube, shortening sequence scan time. For 3D T1 and STIR Cube sequences (Figure 2 and 5) we are able to achieve the desired submillimeter resolution in all planes using HyperSense, reducing scan time which can subsequently be reinvested in resolution.
Figure 1.
With the upgrade to SIGNA™ Premier, T2 FLAIR Cube FatSat sequences provide even greater definition of cortical anatomy and gray/white matter interfaces on thin slice 3D datasets, which improves lesion conspicuity and delineation. (A-B) Discovery™ MR750 with SIGNA™Works (DV26) and 8ch head coil; (C-D) SIGNA™ Premier with SIGNA™Works AIR™ Edition (DV28) and AIR™ 48ch Head Coil.
Figure 2.
Using T1 Cube FatSat post contrast, we can make accurate co-registered comparisons between interval studies. This increases our sensitivity and confidence for identifying early changes in cases of complex skull base disease and perineural extension. This sequence is also of great utility for fusing with radiotherapy planning scans. With the SIGNA™ Premier 3.0T system, we are achieving even better results than previously. (A-C) Comparison images (right + left, top + bottom) demonstrate recurrence and interval progression of residual and previously treated adenoid cystic carcinoma. There is extension along the infraorbital nerve, within the pterygopalatine fossa and along the maxillary branch of the trigeminal nerve via foramen rotundum. The left and top of the comparison images are obtained on the SIGNA™ Premier 3.0T, compared to the Optima™ MR450 1.5T system (right and bottom). The images show how clearly the infraorbital nerve disease is demonstrated at 3.0T, and how this region is more clearly demonstrated than on the older 1.5T scanner.
Software improvements
At Hull, FLAIR Cube is substantially improved on the SIGNA™ Premier and is preferred over 2D FLAIR by our neuroradiologists for all studies, including advanced imaging, dementia, multiple sclerosis and basic morphological imaging. The improvement is due to a number of factors: the SNR improvement from the AIR™ 48ch Head Coil, the new pulse sequence enhancements from SIGNA™Works AIR™ Edition (DV28) and AIR™ Recon, a reconstruction algorithm. An added benefit of the new T2 FLAIR Cube sequence is the improved reformat quality (Figure 1).
Expanded sequences to address complex cases
SCENIC, a coil uniformity algorithm with additional denoising capabilities, delivers more homogenous signal across the entire brain. It also provides denoising to boost image quality for a cleaner appearance when reformatting image data. Intensity correction means we no longer observe surface signal flare off the scalp that previously occurred with other high-element coils.
We use axial DTI at Hull for primary brain tumor patients. With the AIR™ 48ch Head Coil and HyperBand, we can achieve 2 mm isotropic DTI with 40 directions in less than 4 minutes and whole-brain coverage. Additionally, PROGRES, GE Healthcare’s blipped EPI distortion tool, corrects distortions within the DTI data particularly around the skull base and frontal sinus regions.
Figure 3.
In this case, the left frontal lobe glioma is clearly demonstrated on the (A) T2 FLAIR sequence and (B) post-contrast 3D BRAVO. (D) The ASL allows identification of a well demarcated region of higher cerebral blood flow concerning for a focus of higher grade disease/transformation. In our experience we see changes on ASL more clearly than we do on (C) DSC perfusion imaging-rCBV in cases of non-operated gliomas beginning to undergo transformation. Cross referenced to the other sequences, this assists with the treatment planning. (E) The DTI color maps are helpful in suggesting the possibility of a degree of posterior displacement of at least some of the descending motor tracts which assists in surgical planning and informed consent. (F) Multivoxel spectroscopy allows generation of choline/NAA maps which can also be helpful in demonstrating tumour heterogeneity and the likely extent of tumor. This patient underwent awake craniotomy and near total resection.
The axial T2* DSC sequence also benefits from HyperBand and ARC. Using a HyperBand factor of 2 and ARC factor of 2, our enhanced DSC protocol now delivers whole-brain 4 mm slices with a TR=1500 ms compared to the 5 mm slices with a TR=2000 ms that were acquired on the Discovery™ MR750 3.0T. This translates to 67 sampling points instead of 50 measurements for the same scanning duration, delivering higher temporal resolution and allowing better characterization of the signal-time course curve for quantifying perfusion. At Hull, we use BrainStat-AIF to calculate rCBV in primary brain tumors, while the thinner perfusion data also registers with a greater degree of confidence to high spatial resolution morphological sequences.
These improvements all link together. Both DTI and DSC benefit from HyperBand, which links back to the AIR™ 48ch Head Coil and the increased RF channels provided by the SIGNA™ Premier. The entire package supports faster SNR and rich neuroimaging data.
With T2 PROPELLER MB and the AIR™ 48ch Head Coil on SIGNA™ Premier, we can acquire a 512 matrix compared to a 384 matrix on the Discovery™ MR750 with similar scan times thanks to the ability to increase ARC in the phase direction from 2 to 3. The enhancement to PROPELLER suppresses streaking artifacts and works well in the coronal plane. As a result, our adult epilepsy protocol changed from coronal FSE to coronal PROPELLER MB. We found that PROPELLER is now more robust in non- axial scan planes.
PROPELLER Duo is a new diffusion- weighted FSE sequence that delivers reduced background noise and higher SNR than DWI PROPELLER.
“However, most significant is the ability to acquire high-quality, two b-value coronal FSE diffusion (using PROPELLER Duo) data and automatically generate ADC maps, which is useful for acquiring DWI near susceptibility prone areas such as the skull base for cholesteatoma and epidermoid.”
3D ASL is a relatively low SNR technique that benefits from the signal boost provided by the AIR™ 48ch Head Coil, enabling higher diagnostic confidence and increased image consistency. Hull is able to generate higher spatial resolution CBF maps than the standard protocol thanks to SNR gains provided by the coil.
Figure 4.
The PROPELLER Duo sequence allows for axial and coronal DWI acquisitions with automatic generation of ADC maps. This is demonstrated nicely in this patient with a skull base epidermoid. Such detail helps assist in the definition of the anatomical extent of epidermoids/cholesteatomas, particularly when there are also inflammatory changes within the mastoid air cells and middle ear. (A, D) Discovery™ MR750 with SIGNA™Works (DV26) and 8ch head coil; (B, C, E, F ) SIGNA™ Premier with SIGNA™Works AIR™ Edition (DV28) and AIR™ 48ch Head Coil.
Figure 5.
Same patient as Figure 4. Note the skull base epidermoid demonstrated on (A) STIR Cube, (B) T1 Cube and (C) post-contrast T1 Cube FatSat sequences. These are robust sequences which, together, can provide accurate delineation of skull base lesions while also being able to fuse images to radiotherapy planning data sets. The clear delineation of flow voids on T1 Cube and cross reference to the PROPELLER Duo sequence can also be useful in assisting with surgical planning in cases of cholesteatoma, if there is a dehiscent internal carotid artery and cholesteatoma extent is not clearly defined on CT due to additional inflammatory changes.
Figure 6.
This 3-slab Time of Flight (TOF) dataset was acquired from a pediatric patient without sedation or anesthesia. The sparsity of TOF imaging and increased SNR from the AIR™ 48ch Head Coil favor higher compressed sensing factors. The team are routinely able to acquire 720 x 512 matrix sizes using ARC=2 and HyperSense=1.8 in clinically viable times and excellent resolution with a 0.3 x 0.4 x 0.3 mm voxel size. Hull also utilizes FatSat and Magnetization Transfer options on their protocol to further enhance vessel conspicuity.
At Hull, multivoxel spectroscopy is used to assess lesion heterogeneity for low-grade glioma transformation, treatment-induced pseudoprogression and metabolic conditions. To further enhance the B0 homogeneity within the brain, high order shimming hardware provides a means to optimize metabolite linewidths, a metric of spectroscopic quality.
In the example pediatric case, the value of ASL, DSC and 3D spectra are demonstrated when assessing a tumor without gross pathological enhancement (Figure 7). This demonstrates the important information which functional imaging can provide in addition to the morphological pre- and post-contrast imaging.
Figure 7.
Sequences such as (A) 3D FLAIR Cube axial reformat, (D) post-contrast 3D BRAVO and (E) T2 PROPELLER are complemented by the breadth of functional imaging sequences available on the SIGNA™ Premier 3.0T system. In this case (F) CBF derived from 3D PCASL, (B) rCBV calculated from T2* DSC and (C) choline/NAA 3D multivoxel spectroscopy demonstrate high grade features in a region of tumor without gross pathological enhancement.
Conclusion
For us, the SIGNA™ Premier has a superb suite of advanced neuroimaging tools as described above, making it our go-to scanner for advanced problem solving. We can obtain consistent, high-quality results and are confident utilizing the available sequences and technologies.
Our experience with the SIGNA™ Lift program has been excellent. The upgrade to SIGNA™ Premier from Discovery™ MR750 felt seamless in terms of scanner operation and usability, but most importantly, we were able to maintain and often increase the image quality while providing an improved patient experience.