result
1. Desai AP, Mohan P, Nokes B, et al. Increasing Economic Burden in Hospitalized Patients With Cirrhosis: Analysis of a National Database. Clin Transl Gastroenterol. 2019;10(7):e00062. doi:10.14309/ctg.0000000000000062.
2. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019 Jan;70(1):151-171. doi: 10.1016/j.jhep.2018.09.014.
3. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016 Jul;64(1):73-84.
4. Imajo K, Tetlow L, Dennis A, et al. Quantitative multiparametric magnetic resonance imaging can aid non-alcoholic steatohepatitis diagnosis in a Japanese cohort. World J Gastroenterol. 2021 Feb 21;27(7):609-623. doi: 10.3748/wjg.v27.i7.609.
A
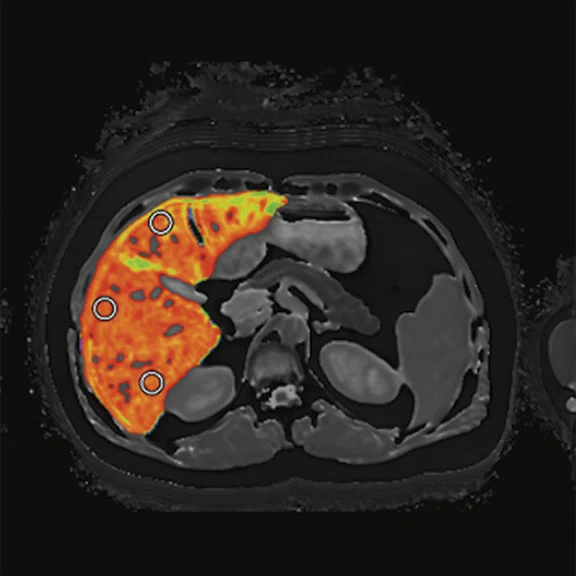
Figure 1.
Using multiparametric MR, LiverMultiScan® generates maps that may indicate the presence of fibro-inflammation, as measured by the metric cT1.
A
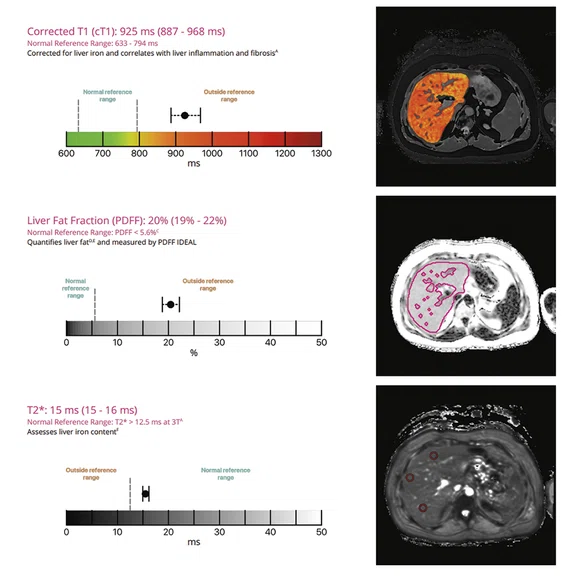
Figure 2.
LiverMultiScan® report of a patient with NASH.
5. Perspectum press release. Available at: https://perspectum.com/news.


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
IN PRACTICE
Quantitative analysis of multiparametric MR to detect early signs of liver disease
Quantitative analysis of multiparametric MR to detect early signs of liver disease
The economic burden of chronic liver disease in the US is estimated at $2.5 billion, with indirect costs up to $10.6 billion and hospitalization costs for patients with cirrhosis at $7.37 billion1. Globally, cirrhosis is the 11th most common cause of death, and liver cancer the 16th most common cause of death2. The rate of liver disease continues to grow, even as more treatments for liver disease become available. Identifying patients who can benefit from early intervention is needed to address this growing health issue.
The economic burden of chronic liver disease in the US is estimated at $2.5 billion, with indirect costs up to $10.6 billion and hospitalization costs for patients with cirrhosis at $7.37 billion1. Globally, cirrhosis is the 11th most common cause of death, and liver cancer the 16th most common cause of death2. The rate of liver disease continues to grow, even as more treatments for liver disease become available. Identifying patients who can benefit from early intervention is needed to address this growing health issue.
Non-alcoholic fatty liver disease (NAFLD) is a disease that ranges from relatively harmless fatty liver to the more severe non-alcoholic steatohepatitis (NASH), where the accumulation of fat causes chronic inflammation and scar tissue (fibrosis). NAFLD is prevalent in approximately 25% of the world’s population, with the highest prevalence in the Middle East and South America3. Advanced stages of the disease lead to cirrhosis and irreversible liver damage, causing cancer and liver failure.
“It is essential to detect NAFLD/NASH early, while treatment options such as lifestyle modification, bariatric surgery and drugs can actually work,” says Catherine Kelly, PhD, MRes, Bsc (Hons), Chief Informatics Officer at Perspectum, a company developing digital technologies and quantitative assessments for the diagnosis and management of liver disease, diabetes and cancer. “It’s therefore critical that clinicians have tools to diagnose, stratify and monitor patients with NAFLD.”
LiverMultiScan® from Perspectum uses multiparametric MR to non-invasively quantify liver tissue to help clinicians evaluate liver diseases. It combines information from multiple parametric maps for a comprehensive analysis of liver tissue. LiverMultiScan® also provides quantitative measures that correlate with the histological features of NAFLD and NASH that are typically acquired from a biopsy.
According to Dr. Kelly, there has been a rapid expansion in the development of pharmacological agents to treat NASH, with the first novel therapies expected to come to market in the next few years. However, treating everyone with NAFLD would be an enormous economic burden on healthcare systems. Therefore, it is important to stratify patients so that those who are most at risk of developing serious NASH can get access to these drugs, before their condition becomes irreversible. Not all drugs will have the same effect on every patient, so monitoring response to therapy will be a critical component of patient management.
“It’s all about getting the right patient on the right treatment for the right duration, using the most robust, precise non-invasive tools available.”
Dr. Catherine Kelly
“LiverMultiScan® has been used as an endpoint in over 30 NAFLD/NASH trials and has shown utility in both diagnosing and monitoring therapy response.”
Currently, patients with liver disease undergo blood tests, ultrasound and biopsy – often repeated – for both diagnosis and management. Liver biopsy is currently the only definitive method to diagnose NASH, yet it measures only 1/50,000th of the liver and is subject to inter-reader sampling variability. The procedure itself is costly and associated with complications that can be severe.
In a recent comparative study, LiverMultiScan® showed the highest agreement with biopsy in both NAFLD and NASH compared to other imaging techniques4.
Early adopter
As one of the largest US radiology groups, US Radiology Specialists® is a partnership of subspecialized radiology groups and outpatient imaging center companies built around a commitment to best-in-class clinical excellence, operations, infrastructure and state-of- the-art technology. One of its imaging center partner companies, Touchstone Imaging, operates centers in six states and provides LiverMultiScan® in five of them. Its Central Austin and San Antonio locations in Texas are using the tool with an Optima™ MR450w 1.5T wide-bore system.
As an organization, Touchstone Imaging has participated in several research studies that included LiverMultiScan®. Approximately 500 patients have undergone exams with this tool across all of Touchstone Imaging’s sites.
“Offering a comprehensive liver imaging/ analysis solution that provides a quantitative report of a patient’s liver health is an important differentiator for our organization,” says Christina McSpedden, Regional Operations Manager for Touchstone Imaging. “LiverMultiScan® allows patients a more cost-effective evaluation and does not include the risks associated with a liver biopsy. It may help prevent patients from having to go through an invasive biopsy procedure and it can also be done without having to use IV contrast.”
The reporting from LiverMultiScan® details the distribution of markers of fat, iron and inflammatory disease that can be used as a tool in the diagnosis of liver disease. The report is turned around quickly to providers, who appreciate the speed and accuracy of the report, she says.
“LiverMultiScan® has given our team access to previously inaccessible specialty physician groups that are looking for cost-effective options to help properly diagnose NASH patients.”
Christina McSpedden
Touchstone Imaging also provides MR elastography and MR proton density fat fraction (PDFF) for a complete MR-based liver health assessment. LiverMultiScan® can help centers capture this new and growing clinical niche.
Implementing LiverMultiScan® doesn’t require additional hardware and is compatible with both 1.5T and 3.0T systems running SIGNA™Works (DV26) or later. The acquisition protocol is loaded onto the scanner and phantom data is acquired for calibration. Images route to Perspectum’s cloud-based service for analysis on the severity of liver disease by examining liver fat, iron and fibroinflammation and how these map across the organ. The completed LiverMultiScan® report from Perspectum can be sent directly into the patient’s chart. Education and training are provided by Perspectum for the imaging center’s staff.
Dr. Kelly adds that two new CPT codes (0648T and 0649T) from the American Medical Association for quantitative multiparametric MR covering LiverMultiScan® will become effective July 1, 20215.
There is increased demand for tools to diagnose, stratify and monitor liver disease, and GE Healthcare offers a complete array of solutions to address this growing need.