result


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
TECH TRENDS
AIR Recon DL from proof-of- concept to clinical product
AIR Recon DL from proof-of- concept to clinical product
Deep learning (DL), a class of machine- learning algorithms, uses neural networks to interrogate data through multiple layers. The algorithm performs a task repeatedly to learn from it, just as a human learns from experience; the more it learns, the better it performs. As such, DL-based products often encompass years of development and testing from research prototyping to commercially cleared product.
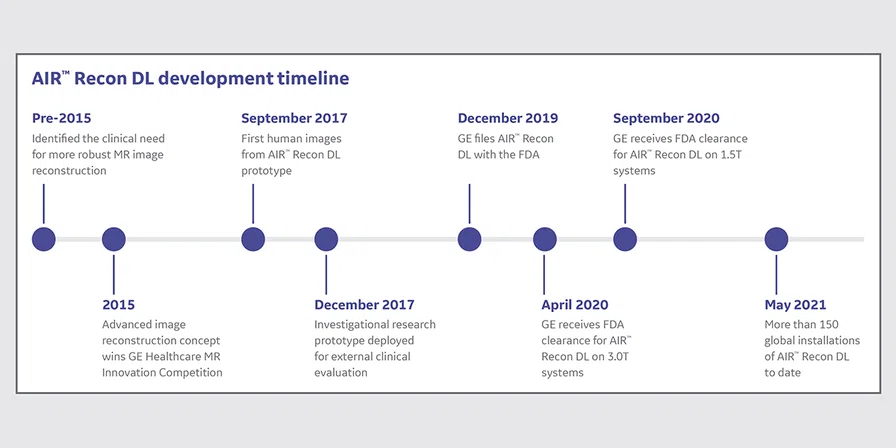
The concept for AIR™ Recon DL began in 2015 when Marc Lebel, PhD, scientist at GE Healthcare, and his colleagues were investigating ways to streamline the MR image reconstruction process and improve image quality by reducing the number of different image manipulation stages. In particular, existing conventional reconstruction methods treated Gibbs ringing as an artifact to be removed.
“The idea was to make the most of our acquired data by reducing what gets discarded from filtering in the image reconstruction. We wanted to actually use Gibbs ringing to help sharpen the images rather than fight to suppress it.”
Dr. Marc Lebel
Key design goals were to move away from inherent deficiencies with existing methods for image reconstruction, including advancing denoising techniques and removing the apodization (raw data) filter for sharper images.
A DL approach
The team looked at other conventional approaches for image reconstruction and examined DL as a potential answer to the clinical need. Several methods were evaluated to achieve this goal, including one that won the 2015 GE Healthcare MR Innovation Competition, which captured the attention of senior MR leadership.
“As DL emerged, we prototyped it,” adds Dr. Lebel, “and it became immediately clear that this was the best option.”
Although the initial prototyping took roughly four weeks, the process to take the DL-based algorithm from a proof-of- concept to a robust commercial product was lengthy. According to Dr. Lebel, the last 5% of the development cycle took 90% of the development time. Most of this was consumed by addressing small issues and generalizing the DL algorithm to many different use cases encompassing resolution, anatomies and imaging options.
After nearly two years of development, the research team led by Dr. Lebel obtained the first internal DL-based reconstructed images in September 2017.
“The image quality was unbelievably good – so good that we had to downgrade the performance of the DL denoising algorithm so that it was more believable,” Dr. Lebel explains. “We immediately knew this was the solution we wanted.”
External feedback with a research prototype
In December 2017, an investigational research prototype was deployed to nearly 20 research partner sites globally across a spectrum of 1.5T and 3.0T GE systems. For the next year, GE collected technical and clinical feedback, and iterated and refined the neural network. This external collaboration was extremely fruitful, as it helped GE improve the DL algorithm while verifying that it was meeting the clinical need. These collaborations also led to over 30 peer-reviewed conference abstracts, podium and webinar presentations, and several full journal articles.
Developing the DL-based reconstruction technique required a substantial overhaul of GE’s reconstruction pipeline infrastructure, including new computing hardware and software interfaces for AI, and a significant commitment of time and resources. The project encompassed long development cycles – each training and evaluation cycle would take several days – with numerous review cycles between the development and applications teams, as well as external clinical experts.
“Our initial clinical feedback was overwhelmingly positive,” he recalls. “It was amazing to see how sites started modifying protocols to achieve faster scans and higher resolution in ways that were previously not possible.”
Dr. Marc Lebel
Reducing scan time was not initially a primary goal; however, it increasingly became apparent that this would be a key benefit of AIR™ Recon DL based on clinical feedback.
The road to regulatory approval
No medical imaging device can become clinically available until after it receives local regulatory approval. GE filed AIR™ Recon DL with the FDA in December 2019 for 3.0T MR systems, receiving clearance in April 2020. The filing for 1.5T systems followed shortly after in August 2020, with clearance one month later. This process required support from many functional units within GE MR.
Sheila Washburn, Senior Digital Product Manager, emphasizes the time it took to develop, test and perform clinical evaluations is not something that should be or can be rushed. It is imperative to ensure that the technique is useful and effective, as well as establish reliability and repeatability to support regulatory clearance and clinical adoption. The team spent a significant amount of time testing and characterizing the performance of the method, including bench tests to understand the AI performance in isolation, system-wide integration tests to ensure the AI worked in the whole system and, of course, clinical studies.
“Regulatory approval definitely did not happen overnight, it was a major undertaking and a relatively new untested technology in medical imaging. Anyone who might imply this is easy, is mistaken.”
Sheila Washburn
“The FDA had great questions and requested clarifications but, fundamentally, our development and testing strategy was solid and accepted, which was the main success in the regulatory process,” Washburn explains. “They didn’t have major issues or concerns requiring a redesign or retest of anything fundamental to the product.”
Prototyping is easy – but developing a robust, regulatory- cleared product that works every time is difficult. Looking back, Dr. Lebel and Washburn see two early, important decisions that were key to the project success. One, focus on the clinical problem to solve and two, test out different technologies and then down sample to what works most efficiently.
“It takes a diverse and committed team to make a product like AIR™ Recon DL succeed,” Dr. Lebel says.
As of May 2021, there are over 150 global installations of AIR™ Recon DL and counting, and clinical feedback continues to be extremely positive.