‡‡ 510(k) pending at the US FDA. Not available for sale in all regions.
‡ 510(k) pending at the US FDA. Not yet CE marked. Not available for sale.
‡ 510(k) pending at the US FDA. Not yet CE marked. Not available for sale.
result


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
NEWS
Three new MR systems launch at RSNA
Three new MR systems launch at RSNA
GE Healthcare is launching several new MR technologies at the annual meeting of the Radiological Society of North America (RSNA), reinforcing our dedication to delivering leading MR capabilities to radiology practices around the world.
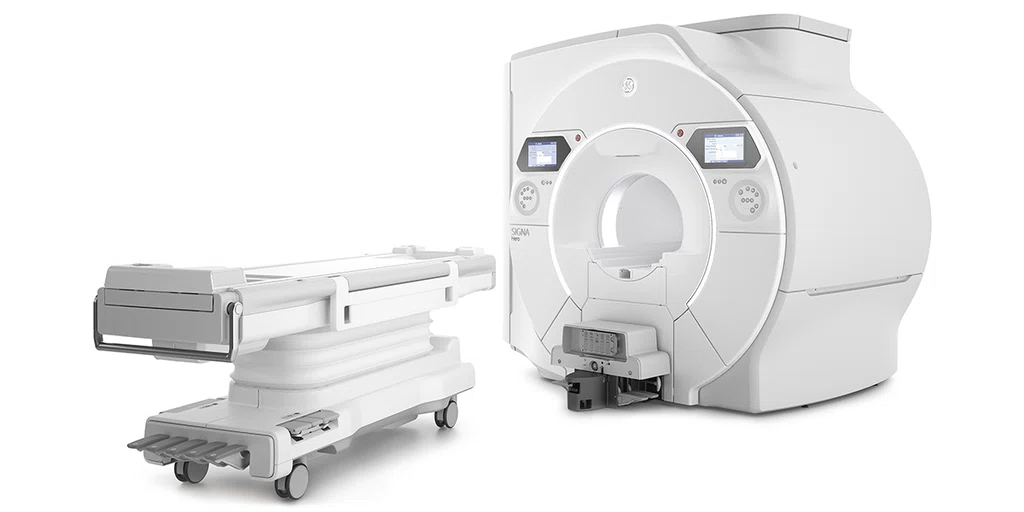
The new SIGNA™ Hero 3.0T‡ is purposebuilt to help clinicians meet every challenge, no matter how big or small. With advancements in deep learning, magnet technology and patient comfort, it enables high patient throughput and complex cases.
The SIGNA™ Artist Evo 1.5T‡ transforms an installed narrowbore system into a 70 cm wide bore system with increased patient weight capacity and new AI-based scanning technology. This upgrade offers affordable access to the latest wide bore capabilities, with fast installation for minimal construction disturbance in the MR suite.
SIGNA™ Prime 1.5T‡‡ makes the MR adoption process simple and seamless. It’s designed to feel as intuitive as any other imaging system you’re already familiar with in your diagnostic imaging department. And it’s built with a simple, environmentally friendly design and future-ready capability. Exclusive features like our new intuitive user experience make SIGNA™ Prime userfriendly for efficient daily operation. Plus, it quickly and easily delivers high-quality images using cuttingedge imaging technology.