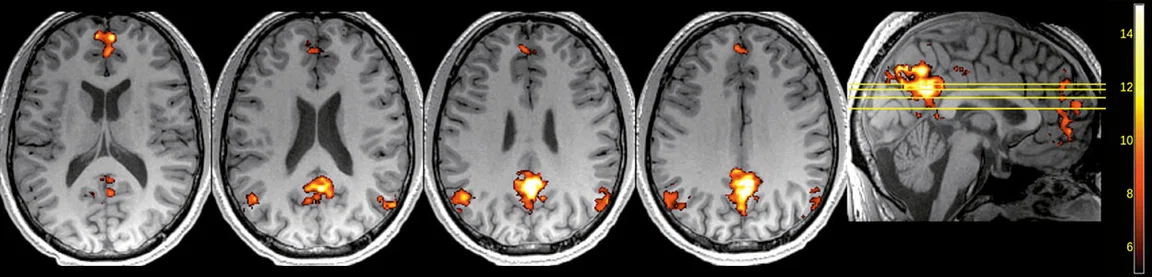
Figure 3.
rs-fMRI study, 2 x 2 x 2 mm, 727 ms TR, 4:34 min.
A
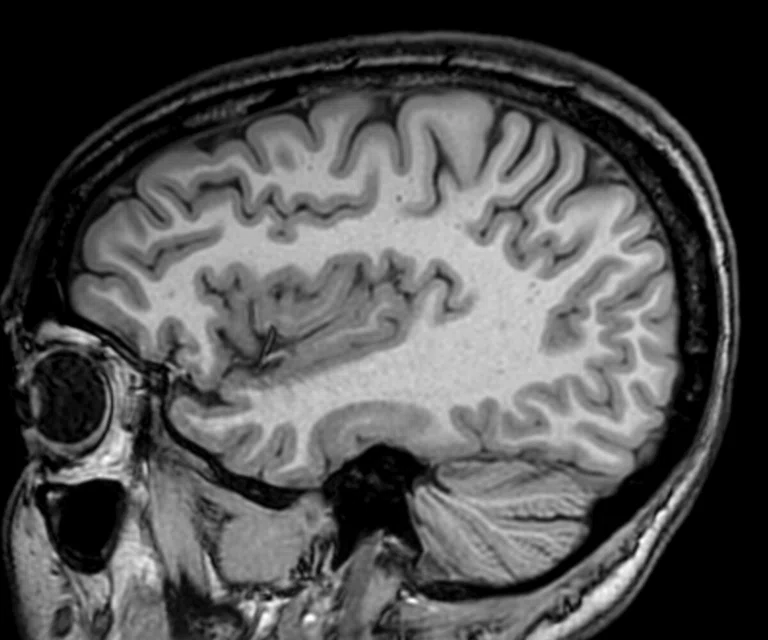
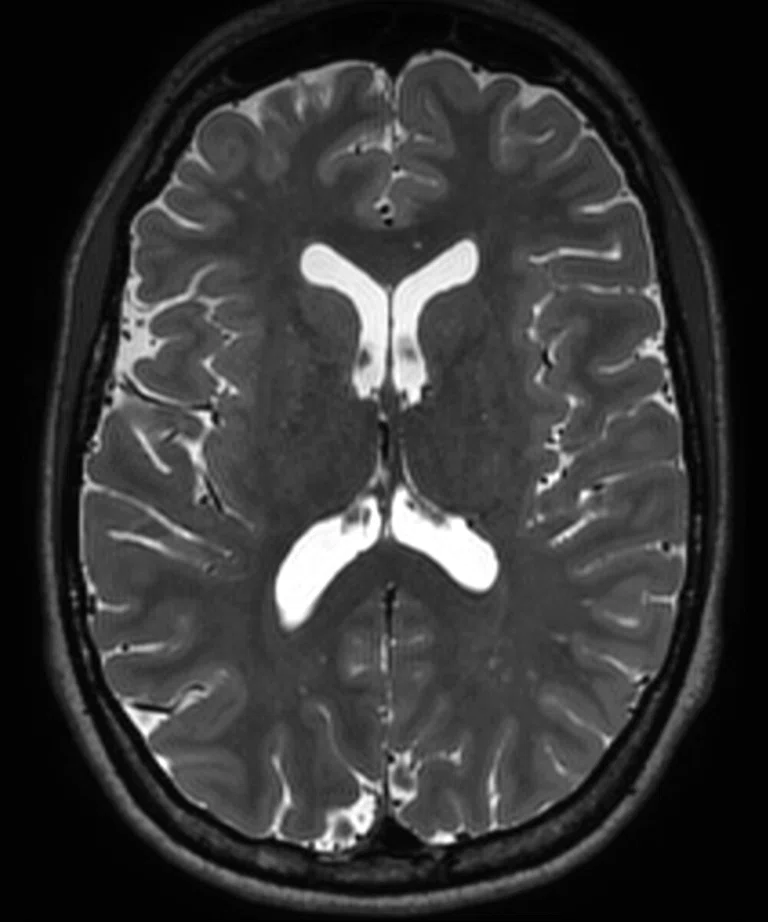
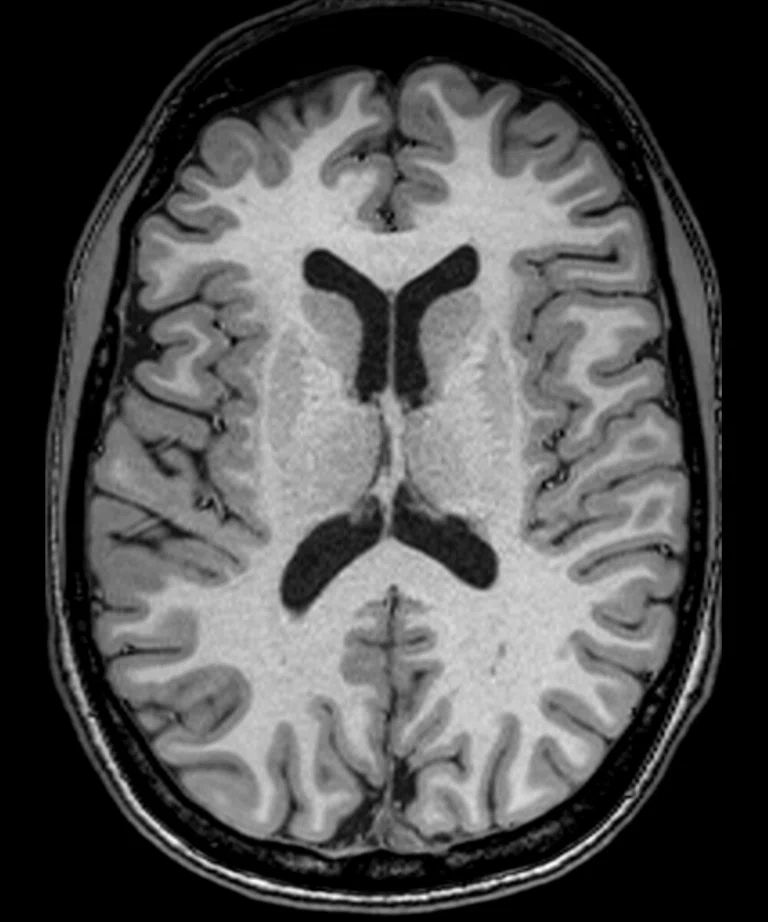
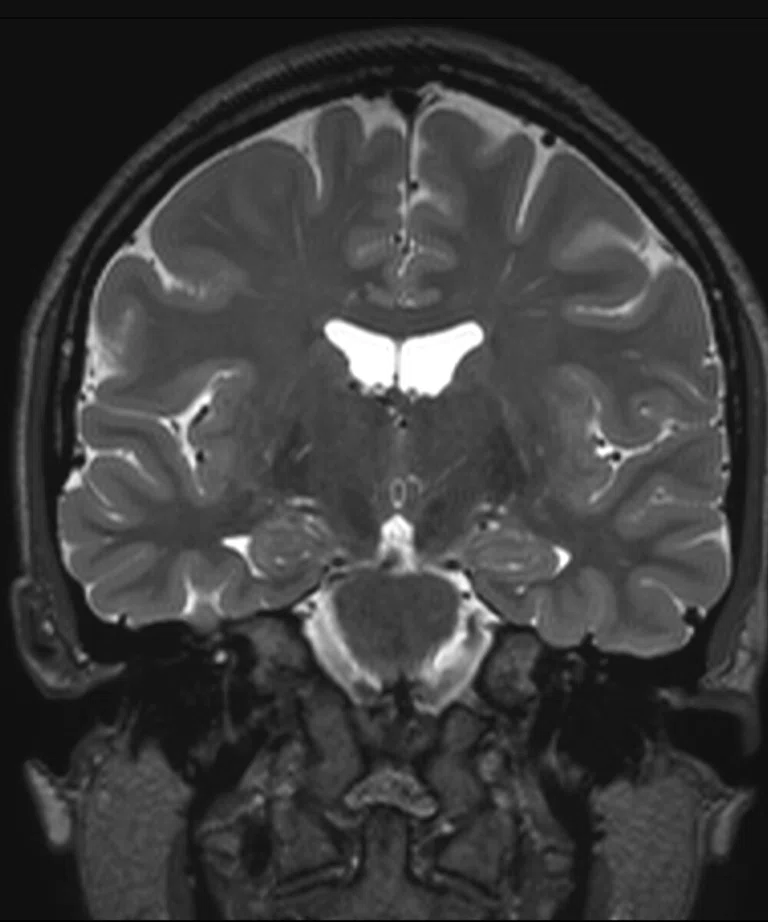
Figure 2.
(A) Sagittal T2 Cube, 0.8 x 0.8 x 0.8 mm, 6:33 min.; (B) sagittal MP-RAGE, 0.8 x 0.8 x 0.8 mm, 4:01 min.; (C, D) axial reformats; and (E-F) coronal reformats.
B
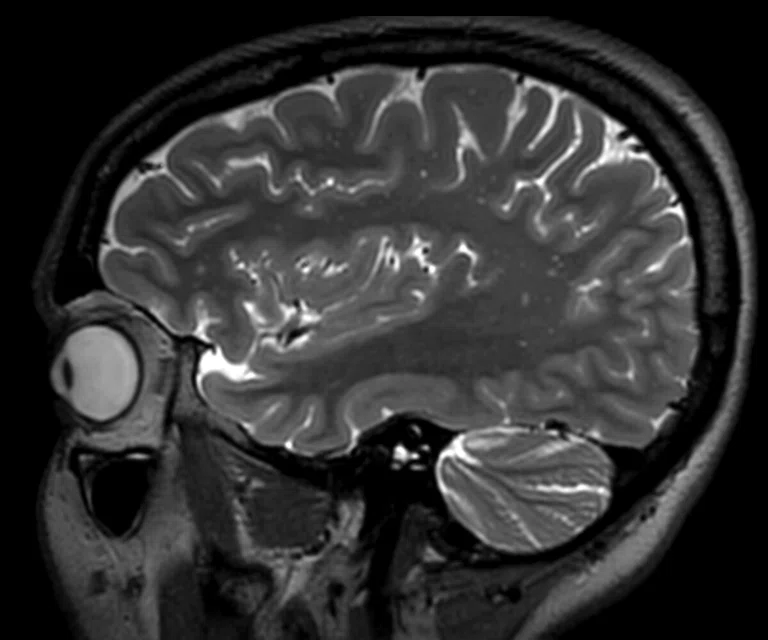
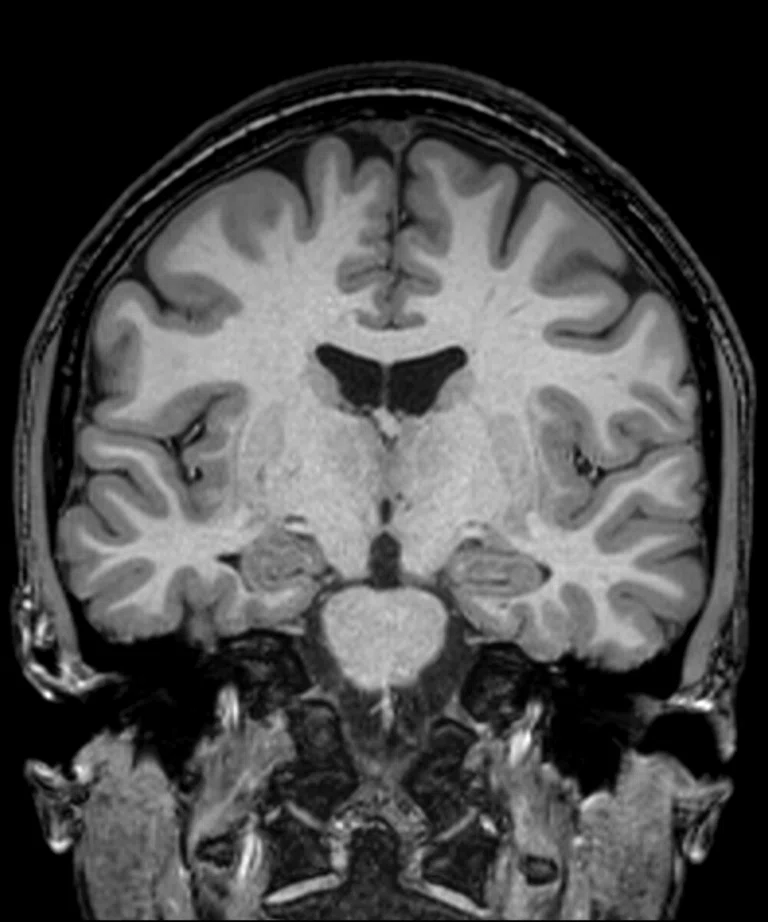
Figure 2.
(A) Sagittal T2 Cube, 0.8 x 0.8 x 0.8 mm, 6:33 min.; (B) sagittal MP-RAGE, 0.8 x 0.8 x 0.8 mm, 4:01 min.; (C, D) axial reformats; and (E-F) coronal reformats.
A
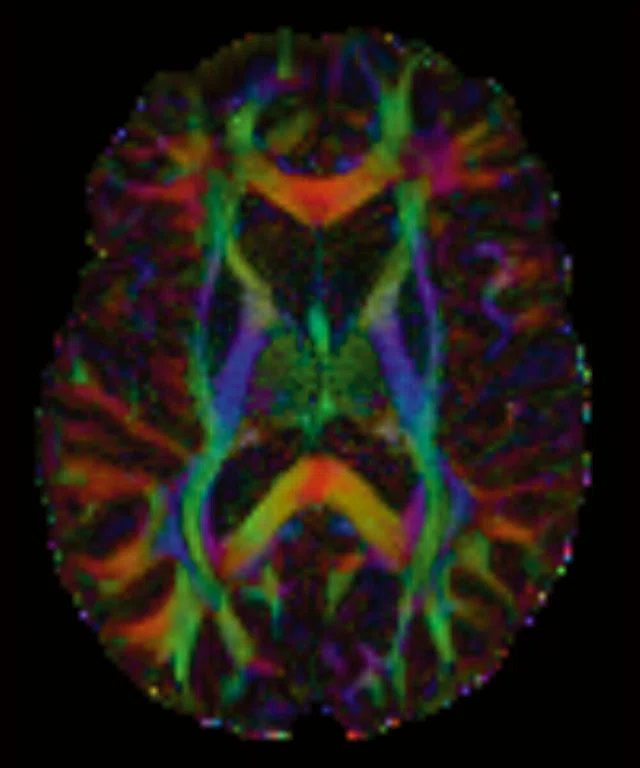
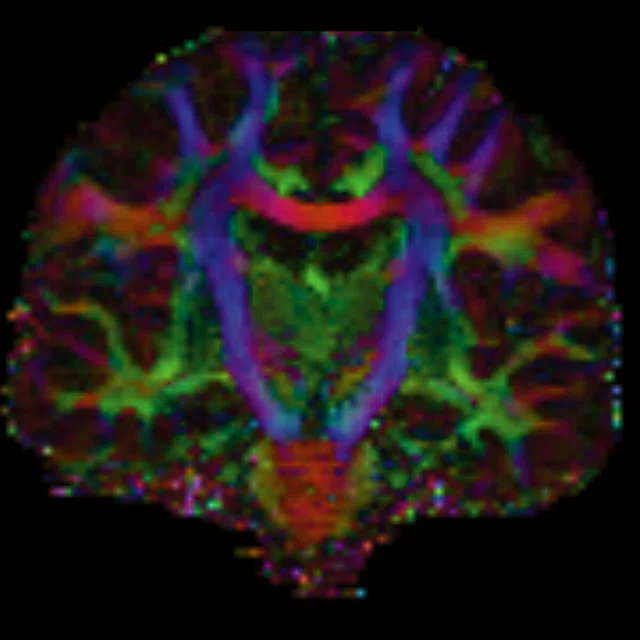
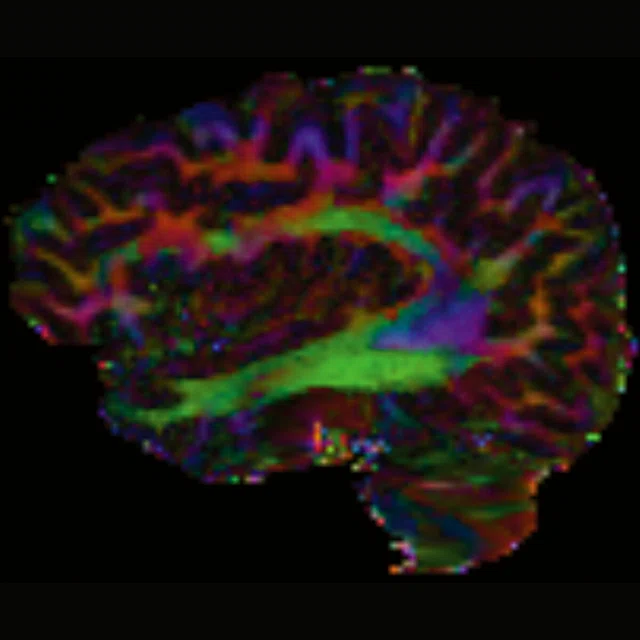
Figure 1.
(A-C) Axial multi-shell DTI acquisition. 76 directions using HyperBand factor of 3, 1.5 x 1.5 x 1.5 mm, 8:13 min.
B
Figure 1.
(A-C) Axial multi-shell DTI acquisition. 76 directions using HyperBand factor of 3, 1.5 x 1.5 x 1.5 mm, 8:13 min.
C
Figure 1.
(A-C) Axial multi-shell DTI acquisition. 76 directions using HyperBand factor of 3, 1.5 x 1.5 x 1.5 mm, 8:13 min.
C
Figure 2.
(A) Sagittal T2 Cube, 0.8 x 0.8 x 0.8 mm, 6:33 min.; (B) sagittal MP-RAGE, 0.8 x 0.8 x 0.8 mm, 4:01 min.; (C, D) axial reformats; and (E-F) coronal reformats.
D
Figure 2.
(A) Sagittal T2 Cube, 0.8 x 0.8 x 0.8 mm, 6:33 min.; (B) sagittal MP-RAGE, 0.8 x 0.8 x 0.8 mm, 4:01 min.; (C, D) axial reformats; and (E-F) coronal reformats.
E
Figure 2.
(A) Sagittal T2 Cube, 0.8 x 0.8 x 0.8 mm, 6:33 min.; (B) sagittal MP-RAGE, 0.8 x 0.8 x 0.8 mm, 4:01 min.; (C, D) axial reformats; and (E-F) coronal reformats.
F
Figure 2.
(A) Sagittal T2 Cube, 0.8 x 0.8 x 0.8 mm, 6:33 min.; (B) sagittal MP-RAGE, 0.8 x 0.8 x 0.8 mm, 4:01 min.; (C, D) axial reformats; and (E-F) coronal reformats.
result


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
TECH TRENDS
A vision for bringing connectome to the clinic
A vision for bringing connectome to the clinic
Ten years have passed since the launch of the Human Connectome Project (HCP), a National Institutes of Health (NIH) funded project to map the human brain and aim to connect its structure to function and behavior. The Medical College of Wisconsin (MCW) has successfully incorporated the Connectome protocols into the SIGNA™ Premier, a 70 cm bore system, and is collaborating with GE Healthcare and other leading research sites to translate neuroscience research to the clinic.
Ten years have passed since the launch of the Human Connectome Project (HCP), a National Institutes of Health (NIH) funded project to map the human brain and aim to connect its structure to function and behavior. The Medical College of Wisconsin (MCW) has successfully incorporated the Connectome protocols into the SIGNA™ Premier, a 70 cm bore system, and is collaborating with GE Healthcare and other leading research sites to translate neuroscience research to the clinic.
In 2014, when the NIH extended the project with a call for proposals to characterize the connectome of specific diseases, MCW was awarded two grants in partnership with the University of Wisconsin: the Epilepsy Connectome Project and the Alzheimer’s Disease Connectome Project.
The demanding diffusion and functional MR protocols in the Connectome initiative also fostered a need for sophisticated reconstruction, including a technique called simultaneous multislice excitation (SMS) that can support high acceleration factors during acquisitions on high-performance gradient hardware.
Over the past decade, there has been a strong effort to acquire a much faster whole-brain resting-state (rs) fMRI study. For example, one snapshot of activation in a whole-brain study can be captured in just 0.75 seconds using high HyperBand (SMS) factors of 6-8.
“We are trying to get inside the physiological window, so that we can map brain activity faster than the body can make changes that confound those measurements,” explains Kevin Koch, PhD, Associate Professor of Radiology & Biomedical Engineering and Director, Center for Imaging Research at MCW.
Similarly, research in diffusion imaging is geared towards multi shell diffusion tensor imaging with a push towards higher b-values in the outer shell. In this case, HyperBand technology is needed to be able to acquire images of the whole brain for many different diffusion encoding directions. The other challenge with these diffusion protocols is that higher b-values need longer diffusion preparation time which increases the echo time (TE), consequently reducing the SNR. This has created a need for more powerful hardware gradients that can enable shorter TEs and, hence, higher SNR at high b-values. Most researchers were performing these studies on specialized research MR systems equipped with high-performance gradients.
“This was the aggressive approach that the Connectome group put together in 2010 on systems that were specifically designed to do this,” Dr. Koch adds. “Being able to do this on a conventional MR system has been the goal and challenge that we have been working on for the last five years.”
MCW successfully obtained both NIH Connectome grants using Discovery™ MR750, a 60 cm commercially available MR system. The deployed protocol utilizes blipped CAIPI HyperBand factor of 8 rs-fMRI with 2 mm isotropic images at 800 ms TR and a 4x blipped CAIPI diffusion tensor imaging (DTI) with 1.5 mm isotropic images and 2 shells at a b-value of 1,000 s/mm2 and 3,000 s/mm2.
Translating research from the bench to the bedside is certainly the ultimate goal, albeit a difficult one. In a clinical environment, patients are not necessarily compliant during the study — a stark contrast to the healthy college-aged students used in the Connectome project. But that has been a focus for the researchers at MCW with the Alzheimer’s Disease Connectome and other projects.
While diffusion tractography is being used as a guiding tool by neurosurgeons at MCW in an intra-operative setting, Connectome protocols are still quite far removed from the clinics. For rs-fMRI, there are still questions in the research and clinical MR communities about how to best interpret the complex and indirect measurements generated by the technology.
“Some of the technology that is being developed with the SIGNA™ Premier, however, may be a step in the right direction,” says Andrew Nencka, PhD, Assistant Professor of Radiology and Associate Director, Center for Imaging Research at MCW. “With the higher temporal resolution acquisitions that we can perform on SIGNA™ Premier, we can better manage patient motion in the fMRI acquisitions.”
As the fourth site in the world to install a SIGNA™ Premier and the first site worldwide to incorporate the Connectome protocol into this system, MCW has taken a leadership role in the GE research community.
While moving the Connectome protocols to a 70 cm bore such as SIGNA™ Premier is not an easy task, a 60 cm bore system also poses its own challenges. Imaging larger patients and having access points for high-end research may be limited at 60 cm. An uncomfortable patient, or one who may barely fit in a 60 cm bore, is more prone to move, which creates image artifacts.
“Having a wide bore MR system that can perform at the level of the 60 cm Discovery™ MR750 is a real enabler for translational research. There is some interesting work being done on lower gradient systems, and if we want to push to the forefront in research, SIGNA™ Premier is an optimal way to do that with high performance, patient access and comfort. The fact that we’ve been able to show parity of a high-end 70 cm system like SIGNA™ Premier with the homogeneity and gradient stability of the 60 cm Discovery™ MR750 is really impressive.”
Dr. Kevin Koch
MCW is working on several other innovative technologies in collaboration with GE, such as multi-echo BOLD ASL that can be used to probe cerebral vascular activity by measuring both BOLD activation and cerebral blood flow contrast simultaneously. It utilizes the multi-band acquisitions developed in the Connectome project and with modest acceleration factors enables whole-brain imaging with the potential to examine physiologic questions like neurovascular coupling, which could be relevant in distinguishing between dementia and vascular dementia.
The big picture, however, is the impact on the GE MR research community that can tap into the knowledge and expertise in protocol development and translational research from MCW and other institutions.
“The GE Collaboration Portal is a great place to tie together all the research being done on GE MR systems worldwide. It is fairly straightforward to get the sequences to other sites that want to use it. There is also a Connectome workgroup that we hope will continue these conversations in exploring neuroscience research.”
Dr. Andrew Nencka