A
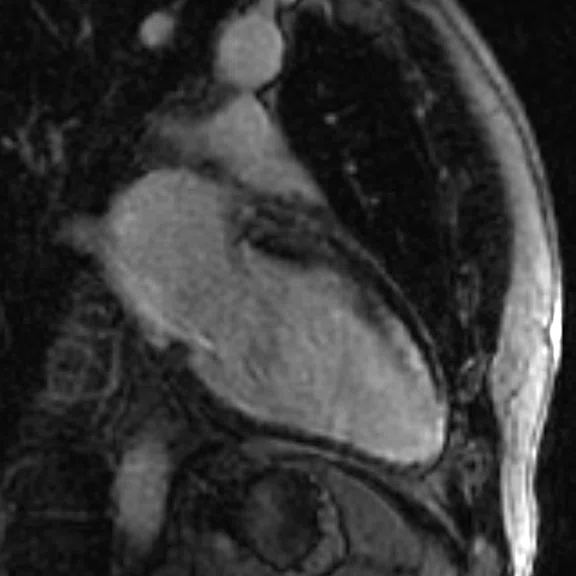
Figure 1.
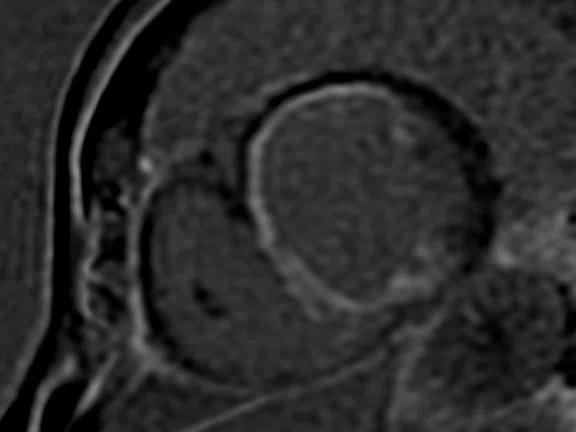
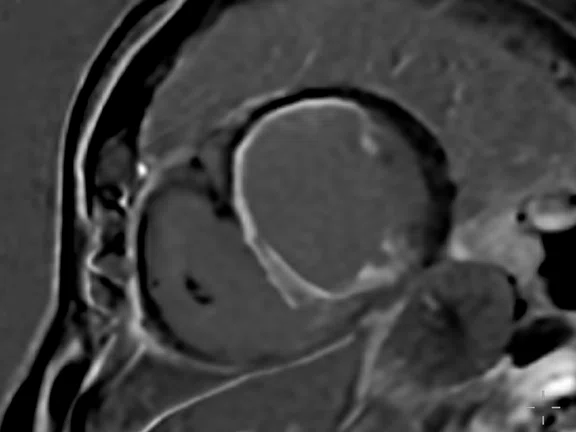
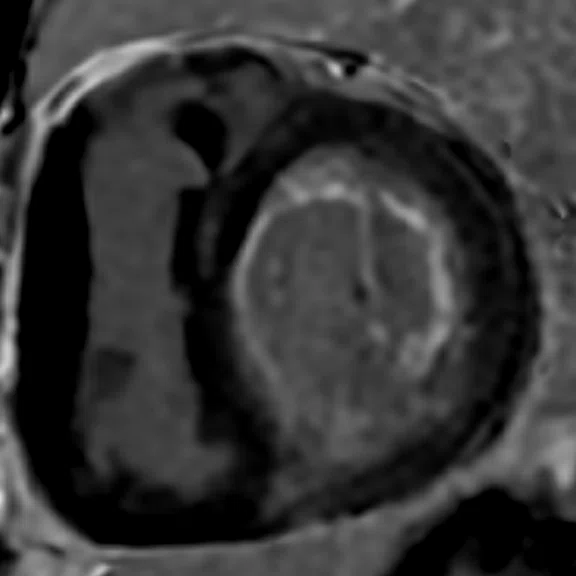
Depicted are two-chamber LGE images with different techniques. (A) Multi-shot bright-blood MDE, (B) single-shot, bright-blood PS MDE with a prototype AIR™ Recon DL (high) and (C) single-shot, dark-blood PS MDE with a prototype AIR™ Recon DL (high).
B
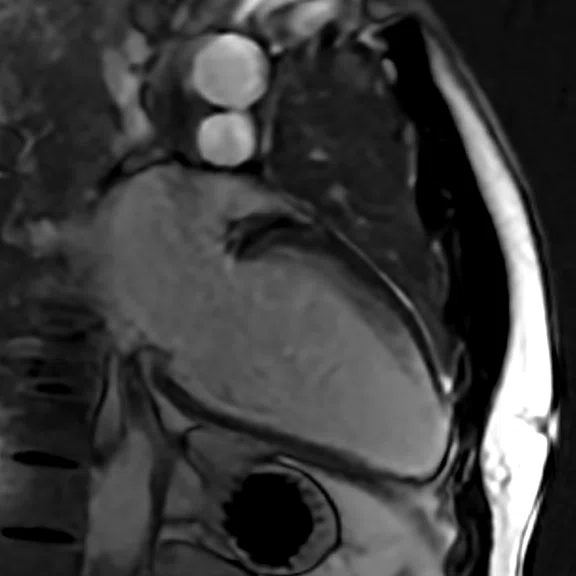
Figure 1.
Depicted are two-chamber LGE images with different techniques. (A) Multi-shot bright-blood MDE, (B) single-shot, bright-blood PS MDE with a prototype AIR™ Recon DL (high) and (C) single-shot, dark-blood PS MDE with a prototype AIR™ Recon DL (high).
C
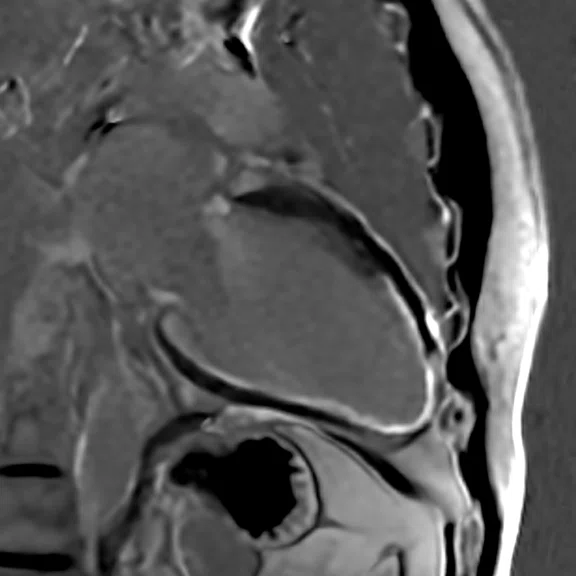
Figure 1.
Depicted are two-chamber LGE images with different techniques. (A) Multi-shot bright-blood MDE, (B) single-shot, bright-blood PS MDE with a prototype AIR™ Recon DL (high) and (C) single-shot, dark-blood PS MDE with a prototype AIR™ Recon DL (high).
A
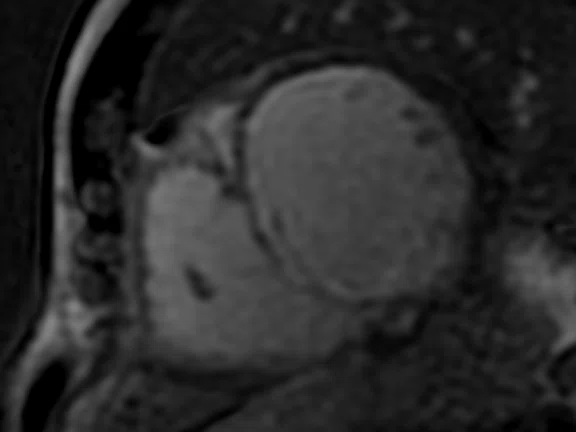
Figure 2.
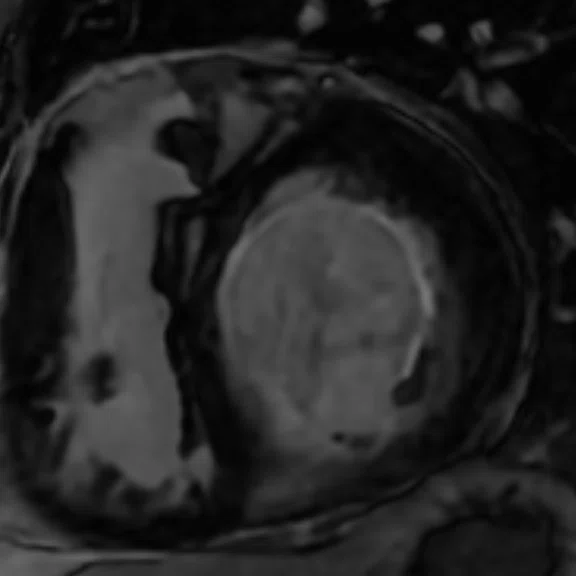
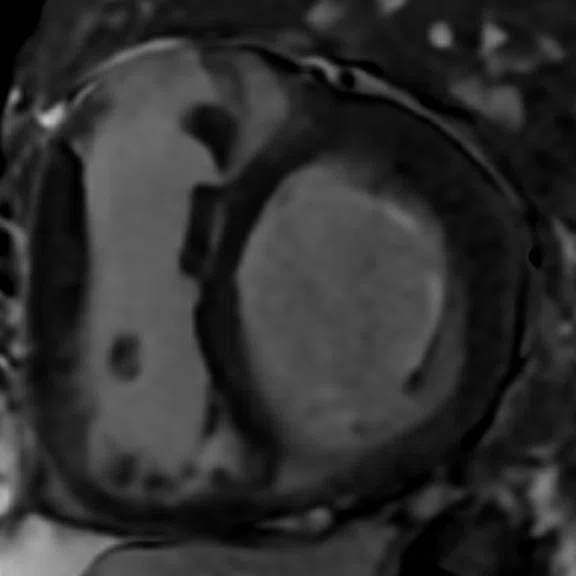
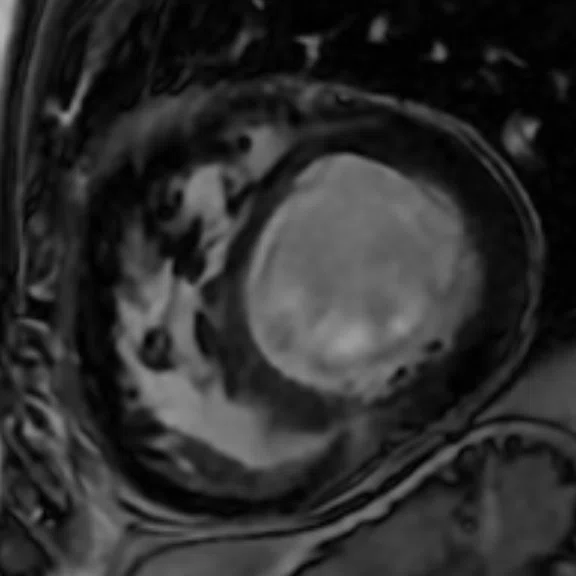
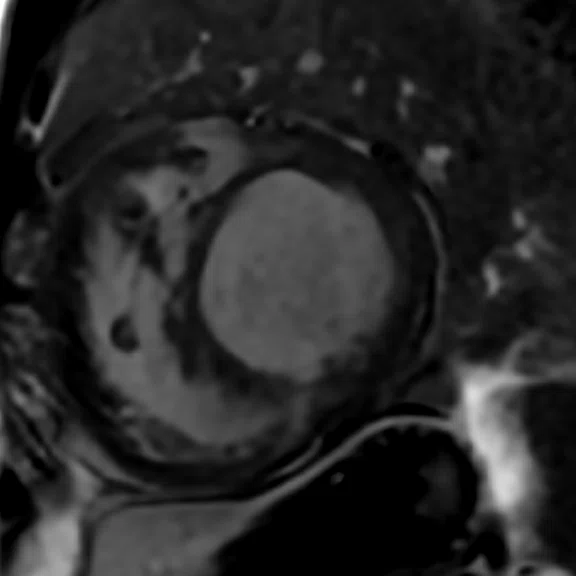
Mid-ventricular short axis single-shot MDE images are shown. (A) Single-shot bright-blood PS MDE, (B) single-shot, dark-blood PS MDE and (C) single-shot dark blood PS MDE with a prototype AIR™ Recon DL (high).
B
Figure 2.
Mid-ventricular short axis single-shot MDE images are shown. (A) Single-shot bright-blood PS MDE, (B) single-shot, dark-blood PS MDE and (C) single-shot dark blood PS MDE with a prototype AIR™ Recon DL (high).
C
Figure 2.
Mid-ventricular short axis single-shot MDE images are shown. (A) Single-shot bright-blood PS MDE, (B) single-shot, dark-blood PS MDE and (C) single-shot dark blood PS MDE with a prototype AIR™ Recon DL (high).
A
Figure 3.
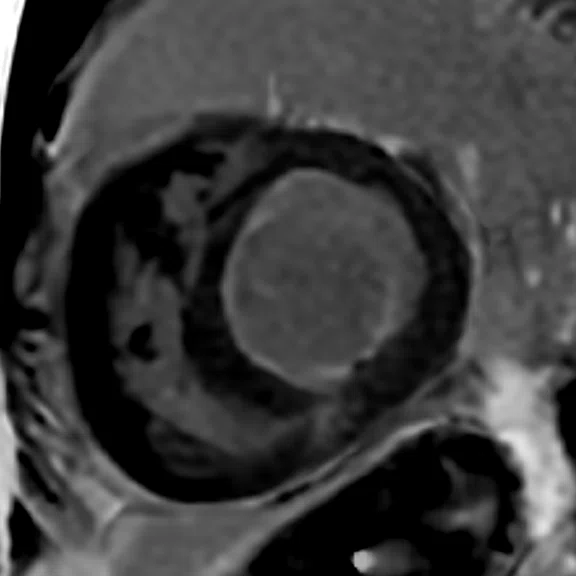
Comparison of (A, D) 2D bright-blood MDE, (B, E) single-shot bright-blood PS MDE and (C, F) single-shot, dark-blood PS MDE. All images are with a prototype AIR™ Recon DL (high). (A-C) Basal short axis MDE images and (D-F) midventricular short axis images.
B
Figure 3.
Comparison of (A, D) 2D bright-blood MDE, (B, E) single-shot bright-blood PS MDE and (C, F) single-shot, dark-blood PS MDE. All images are with a prototype AIR™ Recon DL (high). (A-C) Basal short axis MDE images and (D-F) midventricular short axis images.
C
Figure 3.
Comparison of (A, D) 2D bright-blood MDE, (B, E) single-shot bright-blood PS MDE and (C, F) single-shot, dark-blood PS MDE. All images are with a prototype AIR™ Recon DL (high). (A-C) Basal short axis MDE images and (D-F) midventricular short axis images.
D
Figure 3.
Comparison of (A, D) 2D bright-blood MDE, (B, E) single-shot bright-blood PS MDE and (C, F) single-shot, dark-blood PS MDE. All images are with a prototype AIR™ Recon DL (high). (A-C) Basal short axis MDE images and (D-F) midventricular short axis images.
E
Figure 3.
Comparison of (A, D) 2D bright-blood MDE, (B, E) single-shot bright-blood PS MDE and (C, F) single-shot, dark-blood PS MDE. All images are with a prototype AIR™ Recon DL (high). (A-C) Basal short axis MDE images and (D-F) midventricular short axis images.
F
Figure 3.
Comparison of (A, D) 2D bright-blood MDE, (B, E) single-shot bright-blood PS MDE and (C, F) single-shot, dark-blood PS MDE. All images are with a prototype AIR™ Recon DL (high). (A-C) Basal short axis MDE images and (D-F) midventricular short axis images.
1. Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Eike Nagel. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. 2020 Feb 24;22(1):17.
2. Muscogiuri G, Gatti M, Dell’Aversana S, et al. Image Quality and Reliability of a Novel Dark-Blood Late Gadolinium Enhancement Sequence in Ischemic Cardiomyopathy. J Thorac Imaging. 2020; 35(5):326-333.
3. Holtackers RJ, Chiribiri A, Schneider T, Higgins DM, Botnar RM. Dark-blood late gadolinium enhancement without additional magnetization preparation. J Cardiovasc Magn Reson. 2017; 19(1):64.
4. Holtackers RJ, Van De Heyning CM, Nazir MS, et al. Clinical value of dark-blood late gadolinium enhancement cardiovascular magnetic resonance without additional magnetization preparation. J Cardiovasc Magn Reson. 2019; 21(1):44.
‡Not yet CE marked on 1.5T. Not available for sale in all regions.
5. Lurie PR. Changing concepts of endocardial fibroelastosis. Cardiol Young. 2010; 20(2):115.
6. van der Velde N, Hassing HC, Bakker BJ, et al. Improvement of late gadolinium enhancement image quality using a deep learning-based reconstruction algorithm and its influence on myocardial scar quantification. Eur Radiol. 2020; in press.
result


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
CASE STUDIES
Dark-blood, phase-sensitive myocardial late enhancement for assessing myocardial viability
Dark-blood, phase-sensitive myocardial late enhancement for assessing myocardial viability
by Alexander Hirsch, MD, PhD, Assistant Professor and Principal Investigator of Cardiovascular Magnetic Resonance Imaging, Department of Cardiology/Radiology and Nuclear Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands
For the last two decades, bright-blood late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR) has been the standard noninvasive assessment of myocardial viability in patients with suspected and known ischemia1. A key clinical question is whether the myocardium is still viable. Based on an inversion recovery (IR) pulse sequence performed after gadolinium contrast administration, late gadolinium enhancement can help distinguish ischemic heart disease by nulling the magnetization level of viable myocardium so that its dark appearance is distinguished from scar tissue, which appears bright. However, the adjacent blood pool can have a similar bright signal, making the border between scar and blood difficult to distinguish and in some cases obscured.
This limitation is heightened in cases of thin subendocardial infarction and scarring. Pathology obscured or undistinguishable from the blood pool can result in under reporting of the volume of scar tissue, as well as false negative observations. To overcome these problems, several dark-blood LGE sequences have been developed to improve the contrast between blood and scar, including a novel sequence from GE Healthcare2. However, Holtackers et. al.,3 proposed a simple, elegant dark-blood approach using a standard phase-sensitive (PS) LGE sequence that can easily be implemented in routine clinical care. This dark-blood approach was found to improve detection of ischemic scar and significantly increased total scar burden compared to brightblood LGE. The authors also reported significant improvement in image quality and significantly higher observer confidence4. The only difference between the regular bright-blood PS LGE and dark-blood approach is the setting of the inversion time. For conventional bright-blood PS LGE, the inversion time is set to null viable myocardium, while for dark-blood PS LGE the inversion time is set to null the left ventricular blood pool. Whichever blood pool contrast is desired, the nulling point can be determined by the Cine IR.
In this article, different examples are shown using a single-shot PS LGE (myocardial delayed enhancement, or MDE, on GE MR systems) sequence and Cine IR on the SIGNA™ Artist 1.5T. The inversion time was shortened from nulling remote myocardium to nulling blood pool signal based on the Cine IR images, so that the blood pool appears as black as possible against the infarct that appears bright. The inversion time is set differently for each patient, depending on the amount of contrast, the post-contrast acquisition time and the dynamic of the contrast. Other PS LGE parameters remain unchanged. A prototype of AIR™ Recon DL‡, a deep-learning-based reconstruction algorithm that improves signal-to-noise ratio (SNR) and image sharpness by making use of the raw data to remove image noise and ringing, was also utilized.
Case 1
Patient history
A 60-year-old male without cardiac history presented with out-of-hospital cardiac arrest. An invasive coronary angiogram showed extensive three-vessel disease. CMR was performed to assess viability and showed a dilated left ventricle with poor systolic function.
Results
Images depict extensive subendocardial infarction in both the right coronary artery and the left anterior descending artery territory. The dark-blood PS MDE image (Figure 1C) shows much better delineation between the blood and subendocardial infarction than the bright-blood images (Figures 1A, 1B).
Case 2
Patient history
A 65-year-old male with a history of inferior myocardial infarction was admitted because of heart failure with poor systolic left ventricular function. MR was performed to assess viability after invasive coronary angiogram and helped determine three-vessel disease, including significant left main disease and chronic total occlusion of the right coronary artery and left anterior descending artery. The 2D MDE images were of poor quality because of difficulties with breath-holds (images not shown).
Results
The extensive myocardial infarction is much better visualized with dark-blood PS MDE as shown in Figure 2B, 2C. Also late gadolinium enhancement of the papillary muscle is observed in the dark-blood PS MDE and further enhanced by the prototype AIR™ Recon DL (Figure 2C).
Case 3
Patient history
This is a rare case of restrictive cardiomyopathy with pulmonary hypertension due to endocardial fibroelastosis in a 20-year-old patient with history of congenital subvalvular and valvular aortic stenosis5.
Results
There is a thin layer of endocardial late enhancement visible in the left ventricle that is almost circular. This is best recognized in the dark-blood PS MDE images (Figure 3C). In addition to the endocardial late enhancement, there is also focal late enhancement in the inferior right ventricular insertion point.
Discussion
The dark-blood technique with PS MDE and Cine IR facilitates evaluation of myocardial scarring and viability, especially in cases of subendocardial infarction. It is easy to implement and currently available on most MR scanners and, therefore, can be implemented in clinical practice. As demonstrated by Holtackers et. al., the dark-blood PS MDE is more sensitive for detecting ischemic scar, yet it did not impede the ability to detect thrombus.
A single-shot PS MDE typically results in less SNR and more noise compared to segmented breath-hold techniques. However, the addition of AIR™ Recon DL increases overall image quality by neutralizing the increase in noise and boosting SNR6. While the addition of AIR™ Recon DL is not a requirement for dark-blood PS MDE, it improves a rapidly acquired scan with high image quality. Further, this reconstruction technique helped highlight the infarct in cases of ischemic heart disease with good contrast between the infarct, the blood and the blood pool.
The combination of single-shot PS MDE for dark-blood imaging and AIR™ Recon DL also enhanced our clinical confidence, although diagnoses were unchanged. Understanding how this technique may impact quantification of the infarct is an area that remains to be studied. Therefore, dark-blood, singleshot PS MDE could be used as a rapid tool in addition to standard PS MDE.