A
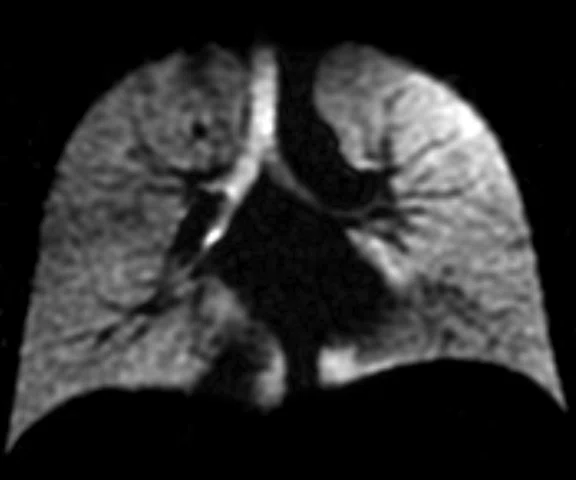
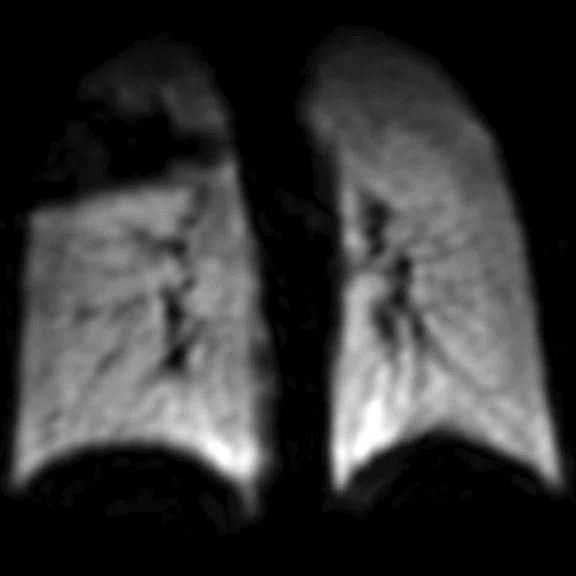
Figure 1.
Pediatric patient with cystic fi brosis. (A) Baseline, ventilation defect percent (VDP) = 3.14%, CVmean = 16.94%, forced expiratory volume in 1 sec. (FEV1) (z-score) = -0.51, LCI = 6.63. (B) Two-year follow-up, VDP = 20.70%, CVmean = 20.15%, FEV1 (z-score) = -0.75, LCI = 8.68.
B
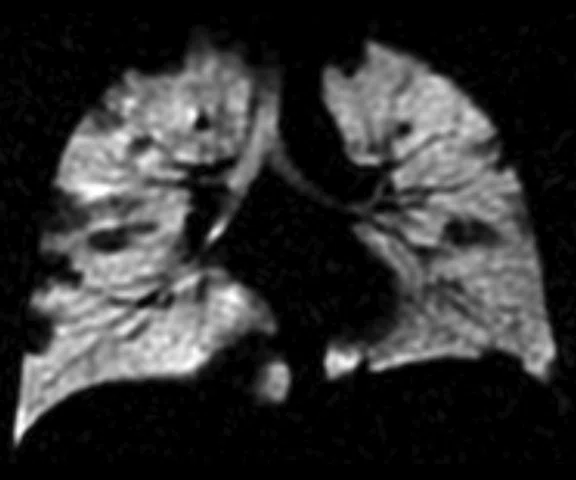
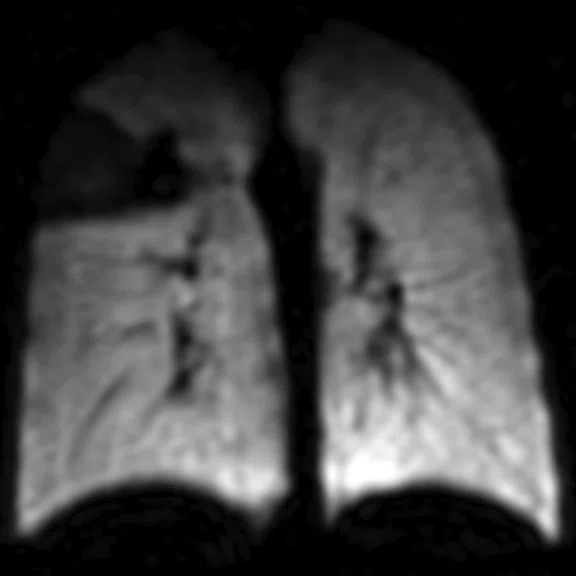
Figure 1.
Pediatric patient with cystic fi brosis. (A) Baseline, ventilation defect percent (VDP) = 3.14%, CVmean = 16.94%, forced expiratory volume in 1 sec. (FEV1) (z-score) = -0.51, LCI = 6.63. (B) Two-year follow-up, VDP = 20.70%, CVmean = 20.15%, FEV1 (z-score) = -0.75, LCI = 8.68.
A
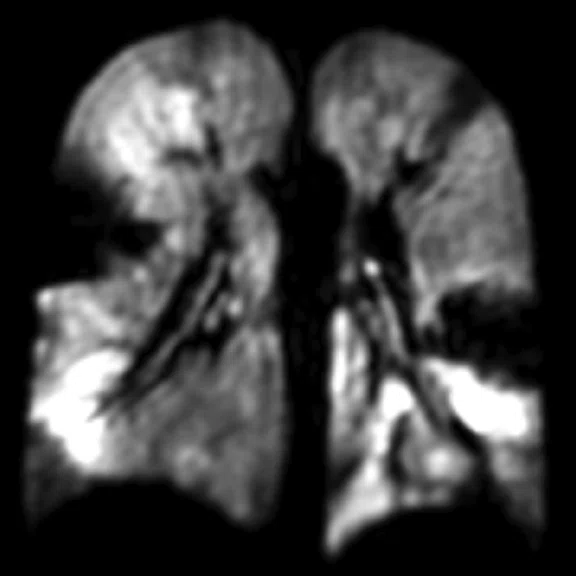
Figure 2.
A 13-year-old cross country runner with decreasing race times with chronic cough and increased sputum production. FEV1 was normal. Patient underwent hyperpolarized 129Xe MR imaging, was diagnosed with non-cystic fi brosis bronchiectasis and treated with IV antibiotics. (A) Baseline, (B) two-weeks after IV therapy.
B
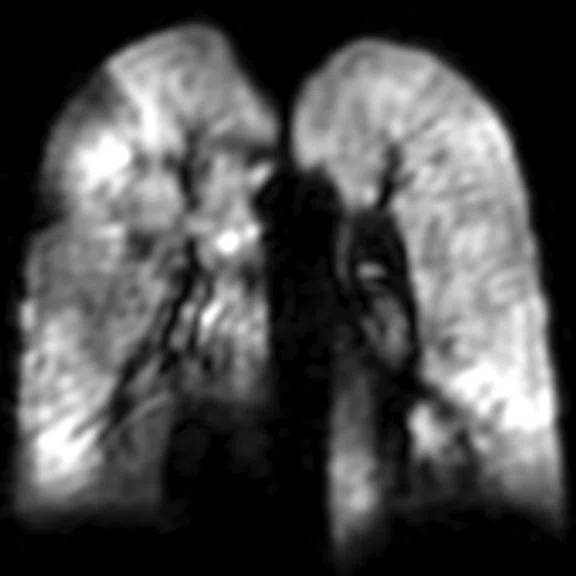
Figure 2.
A 13-year-old cross country runner with decreasing race times with chronic cough and increased sputum production. FEV1 was normal. Patient underwent hyperpolarized 129Xe MR imaging, was diagnosed with non-cystic fi brosis bronchiectasis and treated with IV antibiotics. (A) Baseline, (B) two-weeks after IV therapy.
A
Figure 3.
Cystic fibrosis, 25-minute lung structure-function MR imaging. (A) 129Xe end-inspiratory tidal volume (EIVt); (B) total lung capacity (TLC); (C) ultrashort echo time (UTE); (D) TLC SPGR; and (E) residual volume (RV) SPGR.
B
Figure 3.
Cystic fibrosis, 25-minute lung structure-function MR imaging. (A) 129Xe end-inspiratory tidal volume (EIVt); (B) total lung capacity (TLC); (C) ultrashort echo time (UTE); (D) TLC SPGR; and (E) residual volume (RV) SPGR.
‡ Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
1. Norquay G, Collier GJ, Rao M, Stewart NJ, Wild JM. ^{129} Xe-Rb Spin-Exchange Optical Pumping with High Photon Efficiency. Phys Rev Lett. 2018 Oct 12;121(15):153201.
‡ Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
‡ Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
2. Deppe MH, Parra-Robles J, Marshall H, Lanz T, Wild JM. A Flexible 32-channel Receive Array Combined With a Homogeneous Transmit Coil for Human Lung Imaging With Hyperpolarized 3He at 1.5 T. Magn Reson Med. 2011 Dec;66(6):1788-97.
‡ Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
‡ Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
‡ Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
3. Rao M, Robb F, Wild JM. Dedicated Receiver Array Coil for 1H Lung Imaging with Same-Breath Acquisition of Hyperpolarized 3He and 129Xe Gas. Magn Reson Med. 2015 July;74(1):291-299.
4. Maunder A, Rao M, Robb F, Wild JM. Comparison of MEMS switches and PIN diodes for switched dual tuned RF coils. Magn Reson Med. 2018 Oct;80(4):1746-1753.
7. Marshall H, Horsley A, Taylor CJ, et al. Detection of early subclinical lung disease in children with cystic fibrosis by lung ventilation imaging with hyperpolarised gas MRI. Thorax. 2017 Aug;72(8):760-762.
8. Weatherley ND, Stewart NJ, Chan HF, et al. Hyperpolarised xenon magnetic resonance spectroscopy for the longitudinal assessment of changes in gas diffusion in IPF. Thorax. 2018 Nov 2. pii: thoraxjnl-2018-211851.
9. Horn FC, Marshall H, Collier GJ, et al. Regional Ventilation Changes in the Lung: Treatment Response Mapping by Using Hyperpolarized Gas MR Imaging as a Quantitative Biomarker. Radiology. 2017 Sep;284(3):854-861.
5. Rao MR, Norquay G, Stewart NJ, Hoggard N, Griffiths PD, Wild JM. Assessment of brain perfusion using hyperpolarized 129 Xe MRI in a subject with established stroke. J Magn Reson Imaging. 2019 Feb 18. doi: 10.1002/jmri.26686.
6. Rao MR, Stewart NJ, Griffiths PD, Norquay G, Wild JM. Imaging Human Brain Perfusion with Inhaled Hyperpolarized 129Xe MR Imaging. Radiology. 2018 Feb;286(2):659-665.
‡ Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
‡ Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
‡ Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
‡ Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
‡ Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
2. Deppe MH, Parra-Robles J, Marshall H, Lanz T, Wild JM. A Flexible 32-channel Receive Array Combined With a Homogeneous Transmit Coil for Human Lung Imaging With Hyperpolarized 3He at 1.5 T. Magn Reson Med. 2011 Dec;66(6):1788-97.
1. Norquay G, Collier GJ, Rao M, Stewart NJ, Wild JM. ^{129} Xe-Rb Spin-Exchange Optical Pumping with High Photon Efficiency. Phys Rev Lett. 2018 Oct 12;121(15):153201.
C
Figure 3.
Cystic fibrosis, 25-minute lung structure-function MR imaging. (A) 129Xe end-inspiratory tidal volume (EIVt); (B) total lung capacity (TLC); (C) ultrashort echo time (UTE); (D) TLC SPGR; and (E) residual volume (RV) SPGR.
D
Figure 3.
Cystic fibrosis, 25-minute lung structure-function MR imaging. (A) 129Xe end-inspiratory tidal volume (EIVt); (B) total lung capacity (TLC); (C) ultrashort echo time (UTE); (D) TLC SPGR; and (E) residual volume (RV) SPGR.
E
Figure 3.
Cystic fibrosis, 25-minute lung structure-function MR imaging. (A) 129Xe end-inspiratory tidal volume (EIVt); (B) total lung capacity (TLC); (C) ultrashort echo time (UTE); (D) TLC SPGR; and (E) residual volume (RV) SPGR.
result


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
Dr. First Last
Institure or Hospital
Location

Jim Wild, PhD
University of Sheffield, Sheffield, England
TECH TRENDS
Hyperpolarized gas lung imaging
Hyperpolarized gas lung imaging
The POLARIS group at University of Sheffield, led by Jim Wild, PhD, Professor of Magnetic Resonance Physics and a NIHR Research Professor in Pulmonary Imaging, has been working on hyperpolarized gas lung MR imaging. Their research and technical developments have made a clinical impact on the National Health Service (NHS) practice in the diagnosis and management of patients with asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, interstitial lung disease, lung cancer and pulmonary hypertension.
The POLARIS group at University of Sheffield, led by Jim Wild, PhD, Professor of Magnetic Resonance Physics and a NIHR Research Professor in Pulmonary Imaging, has been working on hyperpolarized gas lung MR imaging. Their research and technical developments have made a clinical impact on the National Health Service (NHS) practice in the diagnosis and management of patients with asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, interstitial lung disease, lung cancer and pulmonary hypertension.
In 2016, the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom approved the use of hyperpolarized gas lung MR imaging for clinical diagnostic referral at the University of Sheffield. Professor Wild now manufactures helium 3 (3He) and xenon 129 (129Xe) for diagnostic MRI for clinical imaging use. Through the NHS, patients are referred to the Royal Hallamshire Hospital, Sheffield Children’s Hospital, and other NHS trusts throughout the UK for hyperpolarized gas and proton lung MR imaging. With MR, they can obtain more insight on a patient’s lung function beyond ventilation.
"The technology allows us to image lung ventilation, gas exchange and microstructure," Professor Wild explains. "We can image the ventilated air spaces and obtain information on lung ventilation changes in obstructed airway diseases, like cystic fibrosis, asthma and COPD."
Also, because xenon is soluble, the gas dissolves in the alveoli in the lungs and goes into the capillaries. This allows measuring and quantifying interstitial changes in fibrotic lung disease and provides insight on ventilation perfusion (VQ) matching. Clinicians can also measure alveoli dimensions by measuring the diffusivity of the gas, which provides metrics on early emphysema changes in smokers and on lung development in infants.
Xenon is an MR-sensitive gas. To image the low concentrations of gas inhaled in the air in the lungs, the spin magnetization needs to be boosted by laser optical pumping. The Sheffield group has built a laser polarizer1‡ that generates highly polarized 129Xe on demand in order to allow high-throughput clinical lung imaging with modest inhaled doses of xenon (500 ml). Using conventional MR scanners (Optima™ MR450w) fitted with a broadband RF amplifier‡, the MR is tuned to a frequency that can detect the hyperpolarized 129Xe. Xenon has a resonant frequency of approximately 25 percent of proton MR; therefore, dedicated RF coils are required.‡
"Our research is geared to making this methodology work on clinically available MR systems," Professor Wild says. "It involves retuning the MR scanner to pick up xenon, designing additional coils to do lung and brain xenon imaging, developing methods to interpret the signals and deriving physiological biomarkers from this kind of imaging. We work closely with GE on the MR hardware and software development for this, particularly in the area of RF coil and pulse sequence developments."
The team has explored the use of parallel imaging methods for hyperpolarized gas MR imaging with a homogeneous field. To accomplish this, they developed a flexible 32-channel receive array‡ to use with an asymmetric birdcage transmit coil‡, which enables high SNR in an accelerated acquisition. The asymmetric birdcage was shown to provide a homogeneous flip angle over the large FOV needed to image the entire lung, while the flexible receive array allowed it to be placed close to the patient’s chest to enable high acceleration factors. The reduction in scan time provided by the use of parallel imaging allowed shorter patient breath-holds.2
TECH TRENDS
Hyperpolarized gas lung imaging
The POLARIS group at University of Sheffield, led by Jim Wild, PhD, Professor of Magnetic Resonance Physics and a NIHR Research Professor in Pulmonary Imaging, has been working on hyperpolarized gas lung MR imaging. Their research and technical developments have made a clinical impact on the National Health Service (NHS) practice in the diagnosis and management of patients with asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, interstitial lung disease, lung cancer and pulmonary hypertension.
The POLARIS group at University of Sheffield, led by Jim Wild, PhD, Professor of Magnetic Resonance Physics and a NIHR Research Professor in Pulmonary Imaging, has been working on hyperpolarized gas lung MR imaging. Their research and technical developments have made a clinical impact on the National Health Service (NHS) practice in the diagnosis and management of patients with asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, interstitial lung disease, lung cancer and pulmonary hypertension.
In 2016, the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom approved the use of hyperpolarized gas lung MR imaging for clinical diagnostic referral at the University of Sheffield. Professor Wild now manufactures helium 3 (3He) and xenon 129 (129Xe) for diagnostic MRI for clinical imaging use. Through the NHS, patients are referred to the Royal Hallamshire Hospital, Sheffield Children’s Hospital, and other NHS trusts throughout the UK for hyperpolarized gas and proton lung MR imaging. With MR, they can obtain more insight on a patient’s lung function beyond ventilation.
"The technology allows us to image lung ventilation, gas exchange and microstructure," Professor Wild explains. "We can image the ventilated air spaces and obtain information on lung ventilation changes in obstructed airway diseases, like cystic fibrosis, asthma and COPD."
Also, because xenon is soluble, the gas dissolves in the alveoli in the lungs and goes into the capillaries. This allows measuring and quantifying interstitial changes in fibrotic lung disease and provides insight on ventilation perfusion (VQ) matching. Clinicians can also measure alveoli dimensions by measuring the diffusivity of the gas, which provides metrics on early emphysema changes in smokers and on lung development in infants.
Xenon is an MR-sensitive gas. To image the low concentrations of gas inhaled in the air in the lungs, the spin magnetization needs to be boosted by laser optical pumping. The Sheffield group has built a laser polarizer1‡ that generates highly polarized 129Xe on demand in order to allow high-throughput clinical lung imaging with modest inhaled doses of xenon (500 ml). Using conventional MR scanners (Optima™ MR450w) fitted with a broadband RF amplifier‡, the MR is tuned to a frequency that can detect the hyperpolarized 129Xe. Xenon has a resonant frequency of approximately 25 percent of proton MR; therefore, dedicated RF coils are required.‡
"Our research is geared to making this methodology work on clinically available MR systems," Professor Wild says. "It involves retuning the MR scanner to pick up xenon, designing additional coils to do lung and brain xenon imaging, developing methods to interpret the signals and deriving physiological biomarkers from this kind of imaging. We work closely with GE on the MR hardware and software development for this, particularly in the area of RF coil and pulse sequence developments."
The team has explored the use of parallel imaging methods for hyperpolarized gas MR imaging with a homogeneous field. To accomplish this, they developed a flexible 32-channel receive array‡ to use with an asymmetric birdcage transmit coil‡, which enables high SNR in an accelerated acquisition. The asymmetric birdcage was shown to provide a homogeneous flip angle over the large FOV needed to image the entire lung, while the flexible receive array allowed it to be placed close to the patient’s chest to enable high acceleration factors. The reduction in scan time provided by the use of parallel imaging allowed shorter patient breath-holds.2
Figure 2.
A 13-year-old cross country runner with decreasing race times with chronic cough and increased sputum production. FEV1 was normal. Patient underwent hyperpolarized 129Xe MR imaging, was diagnosed with non-cystic fi brosis bronchiectasis and treated with IV antibiotics. (A) Baseline, (B) two-weeks after IV therapy.
In collaboration with GE Healthcare, Professor Wild and his team at Sheffield also developed a dedicated 1H receiver array‡ that could improve the SNR that captures both function and structure for use in same-breath acquisition with hyperpolarized gas 3He or 129Xe without having to move the patient or switch coils.3
"The ability to rapidly switch the scanner to image one nucleus and then the next to obtain functional and structural information together from the same coil is very interesting methodologically," he says. "In one project, we have worked closely with GE on ways for rapidly switching the scanner coils between different nuclear frequencies using micro-electro-mechanical switches."4
Since the xenon is inhaled, it also goes into the blood. With the long relaxation time of xenon, Professor Wild and his colleagues have imaged it in other organs.
"We’ve picked up dissolved xenon in the brain and kidneys, so we can monitor cerebral perfusion and kidney perfusion with this technique," he adds.5,6
Evidence-based studies
In a published clinical study of 19 pediatric patients with clinically stable mild cystic fibrosis, hyperpolarized gas ventilation MR was found to be the most sensitive method for detecting abnormalities. The technique also provides detailed regional information on physiological impairment and disease severity, as well as longitudinal changes in lung function.7
"This study demonstrated the ability to follow a given patient and assess their lung function over time, something that cannot be obtained by pulmonary function tests," explains Professor Wild.
Another study examined the use of hyperpolarized 129Xe MR imaging in 18 patients with idiopathic pulmonary fibrosis (IPF). Professor Wild and co-authors concluded that this technique may be sensitive to short-term changes in interstitial gas diffusion in patients with IPF. This is important because prognosticating IPF remains challenging due to the absence of sensitive biomarkers. Existing pulmonary function tests, such as forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO) are insensitive to longitudinal physiological changes that can help a clinician assess disease progression and treatment response.8
In 20 adult asthma patients treated with a bronchodilating agent, two sets of baseline images using hyperpolarized gas ventilation MR were captured prior to treatment and one set after treatment. Treatment response mapping with hyperpolarized gas lung MR imaging provided regional quantitative information on changes in ventilation, complementing current techniques and providing sensitive outcome measures that cannot be obtained with pulmonary function tests.9
"Using the hyperpolarized gas lung MR technique, we could assess treatment response and monitor how specific parts of the lung responded to the inhaled therapy," Professor Wild says. "We have a methodology that is safe, repeatable and robust with added sensitivity to the early signs of lung disease and small changes in response to therapy."
In addition, Professor Wild also believes the technique could be used by pharmaceutical companies in early drug design trials for new therapies to treat asthma, COPD and interstitial lung disease.
He adds, "The ability to monitor change before existing lung function testing is where this technology is really making a difference in respiratory disease."