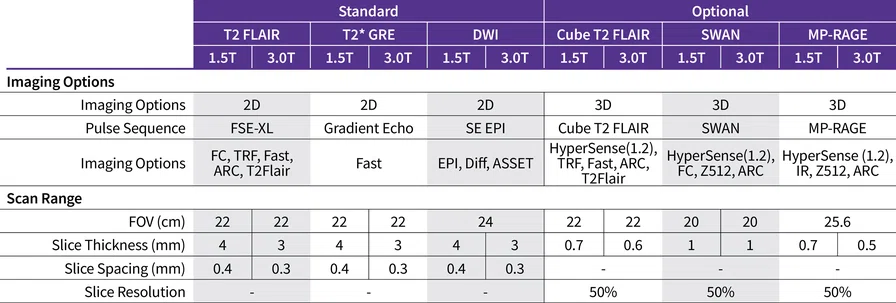
A
Figure 1.
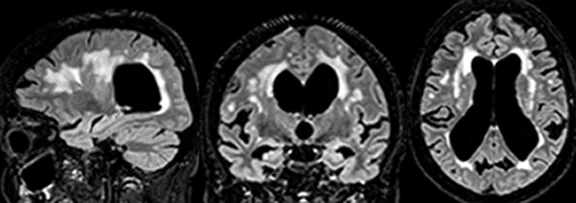
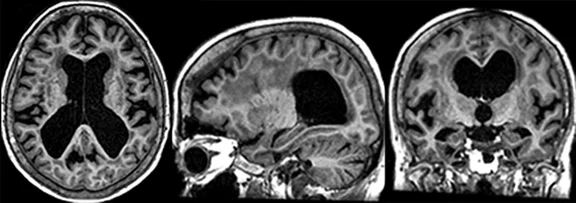
(A) 3D Cube T2 FLAIR with HyperSense acceleration and HyperCube volume localization. (B) 3D MP-RAGE with HyperSense acceleration for T1-weighted imaging. (C) 2D T2 FLAIR with AIR™ Recon DL. (D) Diffusion-weighted EPI with PROGRES distortion correction. (E) PET overlaid on 3D BRAVO. (F) 3D SWAN for susceptibility weighted imaging with magnitude and phase images.
B
Figure 1.
(A) 3D Cube T2 FLAIR with HyperSense acceleration and HyperCube volume localization. (B) 3D MP-RAGE with HyperSense acceleration for T1-weighted imaging. (C) 2D T2 FLAIR with AIR™ Recon DL. (D) Diffusion-weighted EPI with PROGRES distortion correction. (E) PET overlaid on 3D BRAVO. (F) 3D SWAN for susceptibility weighted imaging with magnitude and phase images.
C
Figure 1.
(A) 3D Cube T2 FLAIR with HyperSense acceleration and HyperCube volume localization. (B) 3D MP-RAGE with HyperSense acceleration for T1-weighted imaging. (C) 2D T2 FLAIR with AIR™ Recon DL. (D) Diffusion-weighted EPI with PROGRES distortion correction. (E) PET overlaid on 3D BRAVO. (F) 3D SWAN for susceptibility weighted imaging with magnitude and phase images.
D
Figure 1.
(A) 3D Cube T2 FLAIR with HyperSense acceleration and HyperCube volume localization. (B) 3D MP-RAGE with HyperSense acceleration for T1-weighted imaging. (C) 2D T2 FLAIR with AIR™ Recon DL. (D) Diffusion-weighted EPI with PROGRES distortion correction. (E) PET overlaid on 3D BRAVO. (F) 3D SWAN for susceptibility weighted imaging with magnitude and phase images.
E
Figure 1.
(A) 3D Cube T2 FLAIR with HyperSense acceleration and HyperCube volume localization. (B) 3D MP-RAGE with HyperSense acceleration for T1-weighted imaging. (C) 2D T2 FLAIR with AIR™ Recon DL. (D) Diffusion-weighted EPI with PROGRES distortion correction. (E) PET overlaid on 3D BRAVO. (F) 3D SWAN for susceptibility weighted imaging with magnitude and phase images.
F
Figure 1.
(A) 3D Cube T2 FLAIR with HyperSense acceleration and HyperCube volume localization. (B) 3D MP-RAGE with HyperSense acceleration for T1-weighted imaging. (C) 2D T2 FLAIR with AIR™ Recon DL. (D) Diffusion-weighted EPI with PROGRES distortion correction. (E) PET overlaid on 3D BRAVO. (F) 3D SWAN for susceptibility weighted imaging with magnitude and phase images.
1. World Health Organization. Dementia. Available at: https://www.who.int/news-room/fact-sheets/detail/dementia/?gclid=CjwKCAjwjOunBhB4EiwA94JWsDuQcuA6mHv3hVv5eCdYtvv-Ztp7aflepHJqE_rSRzzZiLy1WvSDghoC1usQAvD_BwE
2. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023;388(1):9-21.
3. Pang M, Zhu L, Gabelle A, et al. Effect of reduction in brain amyloid levels on change in cognitive and functional decline in randomized clinical trials: An instrumental variable meta-analysis. Alzheimers Dement. 2023;19(4):1292-1299.
4. Cogswell PM, Barakos JA, Barkhof F, et al. Amyloid-Related Imaging Abnormalities with Emerging Alzheimer Disease Therapeutics: Detection and Reporting Recommendations for Clinical Practice. AJNR Am J Neuroradiol. 2022;43(9):E19-E35. doi:10.3174/ajnr.A7586.
‡Not CE marked. Not available for sale in all regions.
result


PREVIOUS
${prev-page}
NEXT
${next-page}



Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
Tech Trends
Optimizing the ARIA imaging protocol on GE HealthCare MR systems
Optimizing the ARIA imaging protocol on GE HealthCare MR systems
By Steve Lawson, RT(R)(MR), Associate Director, Global MR Clinical Marketing, Anja Fofana, MTRA, Clinical Marketing Leader, and Suchandrima Banerjee, PhD, Senior Director, Global MR, GE HealthCare
In July 2023, the US Food and Drug Administration gave traditional (full) approval to an amyloid beta-directed antibody for the treatment of Alzheimer’s disease (AD) for the first time, two years after another amyloid beta-directed monoclonal antibody received accelerated approval. With such therapies entering the clinic, the potential to improve patient care and possibly slow disease progression is enormous. More than 6 million Americans are living with AD, and more than 55 million people worldwide have dementia.1
This family of treatments target the pathology of this disease by reducing the beta-amyloid plaques that typically accumulate in the brains of AD patients. Clinical studies have demonstrated
that removing beta-amyloid from the brain reduces cognitive and functional decline in patients living with early AD.2,3
However, monoclonal antibody treatments that target amyloid plaques carry a risk of known side effects — Amyloid Related Imaging Abnormalities (ARIA). ARIA is classified into two categories: ARIA-E, representing edema and/or effusion, and ARIA-H, representing hemorrhage. ARIA is caused by a treatment-related inflammatory response leading to increased vascular permeability in which blood and fluids leak into surrounding tissues. Patients receiving either of these two treatments for early AD will require MR for eligibility assessment and will then routinely undergo safety monitoring with MR as they receive therapy.
A group of neurodegenerative disease experts from the American Society of Neuroradiology (ASNR) published recommendations around MR protocols for ARIA monitoring, based on observations and findings from clinical trials.4 However with the introduction of such therapies into the clinic, it is clear that more guidance is needed to implement standardized imaging and reporting of ARIA findings in the clinical workflow, as well as to cope with the increase in brain MR examinations that is expected, given the percentage of the population afflicted with mild cognitive impairment (MCI) or AD.
Tammy Benzinger, MD, PhD, Professor at Washington University School of Medicine in St. Louis and Chief of MRI and a neuroradiologist at the Mallinckrodt Institute of Radiology, has been leading the ASNR efforts. Dr. Benzinger says, “The ASNR Study Group on ARIA, AD and dementia was formed in 2021 to help prepare neuroradiologists for the interpretation of brain MRs for patients with dementia, and in particular, for whom monoclonal antibody therapy for AD was under consideration. We have been very excited to work with GE HealthCare and other corporate partners to develop streamlined protocols for MR scanning for these patients. The proposed ARIA MR protocols are designed to be under 15 minutes and provide the three key sequences, FLAIR, HEMO and diffusion, in a standardized format. This will allow patients to access high-quality, reproducible imaging in both hospital and outpatient settings.”
GE HealthCare was one of several MR manufacturers and post-processing software vendors to work with the ASNR to develop an ARIA-specific protocol that meets the base requirements of the ASNR consensus criteria for implementation on any of the company’s fleet of commercial scanners. In addition, advanced imaging capabilities are available that can reduce scan time and/or increase SNR and image consistency to help improve diagnostic quality of the images, as well as patient throughput and patient comfort.

To access the complete protocol, visit:
signapulse.gehealthcare.com/aria-protocol-1.5t
signapulse.gehealthcare.com/aria-protocol-3.0tLet’s start with the ASNR base protocol. ARIA-E has an imaging appearance similar to vasogenic edema and may present as either parenchymal edema or sulcal effusion, or both. It is best visualized with a T2 FLAIR sequence.
In clinical trials, ARIA-H was detected on T2* gradient recalled echo (GRE) images. 3D FLAIR and 3D susceptibility weighted images could provide better resolution and sensitivity, however, these would have to be calibrated against the 2D FLAIR and 2D GRE T2* scans used to study ARIA in the clinical trials. Additionally, a 3D T1 sequence such as MP-RAGE may be recommended as part of the baseline scan to estimate brain atrophy.
To differentiate ARIA from any infarcts (acute/subacute or incidental), diffusion-weighted imaging (DWI) is employed. A standard axial 2D DWI sequence is recommended.
Now, let’s look at how GE HealthCare MR users can further enhance their base ARIA protocol. The advancements described below are all available in our latest software version, MR 30.1 for SIGNA™. And depending on the specific scanner capabilities, this ARIA protocol can be built on legacy equipment.
AIR™ Recon DL is GE HealthCare’s pioneering deep-learning-based reconstruction algorithm that removes noise and ringing from raw images, improving SNR to sharpen images up to 60% and enabling up to 50% faster scan times. AIR™ Recon DL has recently been expanded to include motion-insensitive PROPELLER and 3D imaging capabilities, making it compatible with nearly all sequences in the ARIA protocol.
PROPELLER is designed to reduce the effect of patient voluntary and physiologic motion (breathing, flow, peristalsis), and reduce magnetic susceptibility artifacts. It uses a rotating blade k-space filling pattern that, compared to Cartesian methods, is inherently less sensitive to motion, such as CSF and blood flow, breathing, patient tremor or voluntary movements. It is compatible with T2 FLAIR and axial DWI for brain imaging. While it is not part of the core ARIA protocol, this motion-insensitive option might be used by sites in cases of scan failures due to motion.
AIR™ Recon DL can be used with 3D FLAIR and 3D T2 FSE acquisitions to obtain high-quality, high-SNR and reliable images in shorter scan times if a practice decides to utilize 3D sequences.
PROGRES improves diffusion image quality with distortion correction, cleaning up unwanted artifacts for DWI/DTI images.In 3.0T MR imaging, the AIR™ 48-channel head coil, developed as part of the GE-NFL Head Health Initiative, is designed for high SNR brain imaging and high patient population compatibility so that it can fit 99.9% of patients. The coil topology is designed for optimum parallel imaging acceleration performance.
Patients receiving therapy will be imaged at multiple time points and, therefore, consistent image prescription might be a challenge. AIR x™ is another GE HealthCare deep-learning-based innovation that automatically detects the anatomy and prescribes MR slices to deliver consistent results regardless of pathology, patient positioning or technologist. This approach reduces sources of variation and ensures that patients undergoing repeat ARIA exams have consistent, reproducible scans each time. This supports neuroradiologists who need to compare and quantify results with confidence.
Users of GE HealthCare’s industry-leading PET/MR who simultaneously scan with PET and MR know the value of co-registered PET and MR images for AD. PET measures the accumulation of beta-amyloid plaque, which cannot be detected with MR or CT. Beta-amyloid plaque deposition may begin as early as 10 to 15 years before cognitive impairment symptoms. GE HealthCare also has a PET tracer that binds to beta-amyloid and can be used as a critical AD diagnostic tool.
C
D
E
F
Figure 1.
(A) 3D Cube T2 FLAIR with HyperSense acceleration and HyperCube volume localization. (B) 3D MP-RAGE with HyperSense acceleration for T1-weighted imaging. (C) 2D T2 FLAIR with AIR™ Recon DL. (D) Diffusion-weighted EPI with PROGRES distortion correction. (E) PET overlaid on 3D BRAVO. (F) 3D SWAN for susceptibility weighted imaging with magnitude and phase images.
In addition to the technologies listed above, GE HealthCare MR and PET/MR users may also utilize the following tools to customize and accelerate ARIA imaging:
- HyperSense delivers a compressed-sensing boost to image acceleration for faster and higher resolution acquisitions.
- PROMO is a real-time prospective motion correction technique that improves image quality in the presence of patient motion, with minimal scan time penalty, and without external cameras or markers.
- CortexID Suite is a fully automated, post-processing solution to quantify PET beta amyloid and FDG scans acquired on PET/MR and other hybrid PET scanners. CortexID may aid in the interpretation of PET studies on patients evaluated for cognitive impairment or other causes of cognitive decline and is an adjunct to other diagnostic evaluations.
- Growing family of post-processing applications that enable access to the latest, cutting-edge capabilities for quantitative report generation, including atrophy reports with Quantib® Brain, NeuroQuant® and icobrain-dm.
- Patient-specific, personalized MR attenuation correction (ZTE MRAC) for the head in SIGNA™ PET/MR using Zero-Echo Time MR.
- MotionFree Brain for SIGNA™ PET/MR‡ — a completely data-driven, retrospective motion correction technology requiring no external hardware and having no impact on the clinical routine.
As ARIA imaging is implemented into the clinic and our collective understanding of this side effect is augmented, GE HealthCare will continue to work with its customers and medical societies, such as ASNR, to further standardize, harmonize and streamline the protocol.
DOWNLOAD ARTICLE HERE