A
Figure 1.
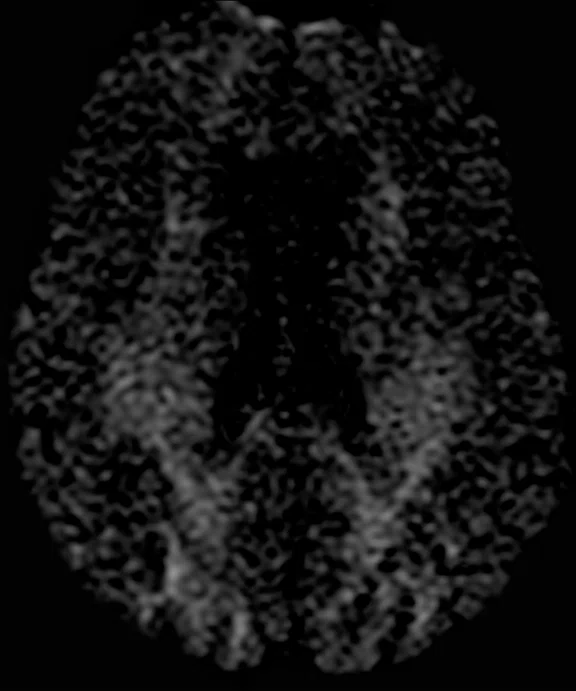
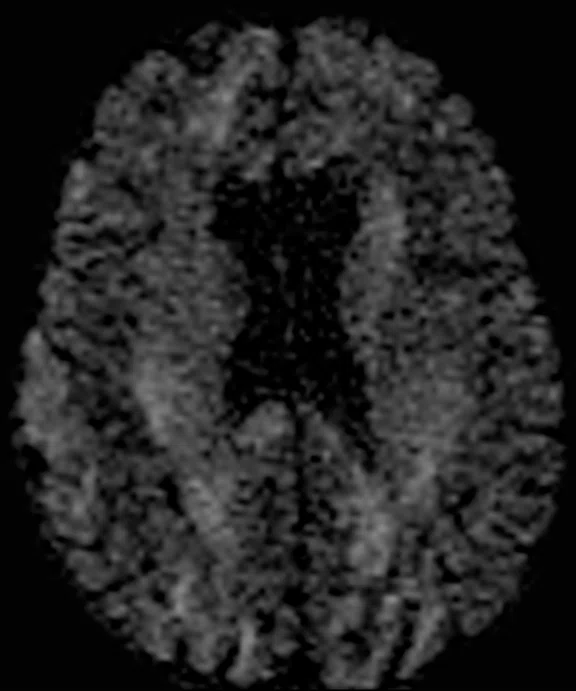
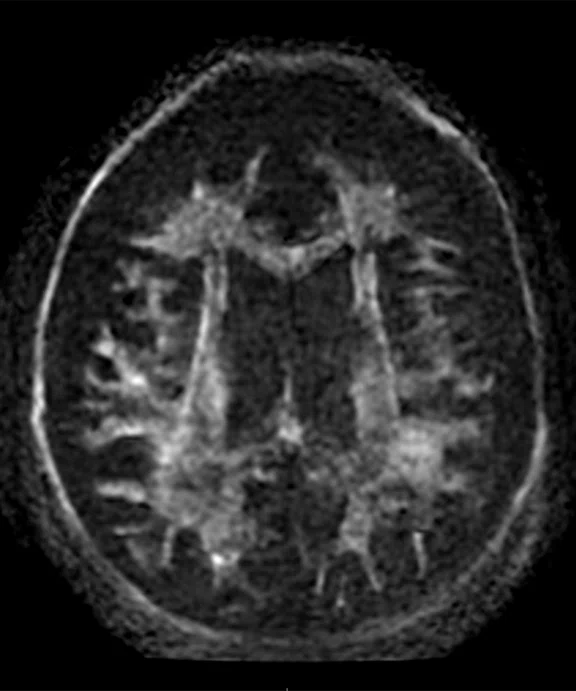
Comparison of single-shot diffusion weighted images with b3000 and AIR™ Recon DL on (A) SIGNA™ Premier 3.0T using a GE HealthCare 48-ch head coil and (B) MAGNUS 3.0T using a Nova Medical 32-ch coil. Both data sets were acquired with the same parameters except TE, which was 71 ms for SIGNA™ Premier and 36 ms for MAGNUS. Note the higher SNR and reduced distortion in the MAGNUS image. (C) Diffusion tensor b30,000, TE=55 msec. with AIR™ Recon DL acquired on MAGNUS.
B
Figure 1.
Comparison of single-shot diffusion weighted images with b3000 and AIR™ Recon DL on (A) SIGNA™ Premier 3.0T using a GE HealthCare 48-ch head coil and (B) MAGNUS 3.0T using a Nova Medical 32-ch coil. Both data sets were acquired with the same parameters except TE, which was 71 ms for SIGNA™ Premier and 36 ms for MAGNUS. Note the higher SNR and reduced distortion in the MAGNUS image. (C) Diffusion tensor b30,000, TE=55 msec. with AIR™ Recon DL acquired on MAGNUS.
C
Figure 1.
Comparison of single-shot diffusion weighted images with b3000 and AIR™ Recon DL on (A) SIGNA™ Premier 3.0T using a GE HealthCare 48-ch head coil and (B) MAGNUS 3.0T using a Nova Medical 32-ch coil. Both data sets were acquired with the same parameters except TE, which was 71 ms for SIGNA™ Premier and 36 ms for MAGNUS. Note the higher SNR and reduced distortion in the MAGNUS image. (C) Diffusion tensor b30,000, TE=55 msec. with AIR™ Recon DL acquired on MAGNUS.
A
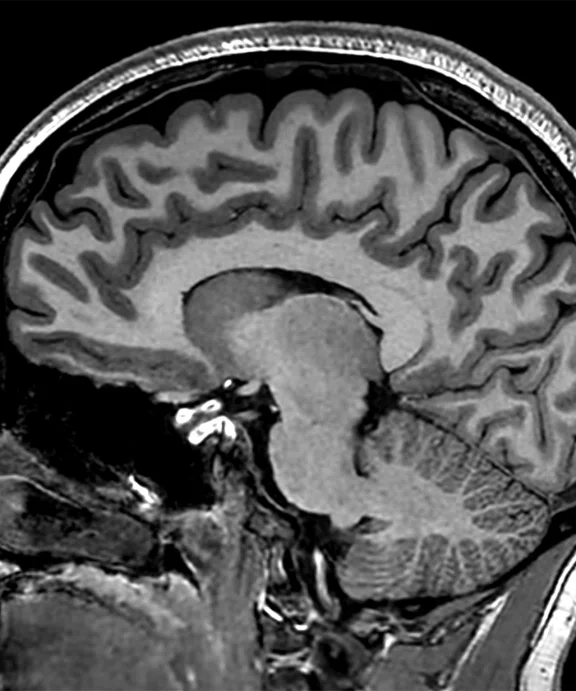
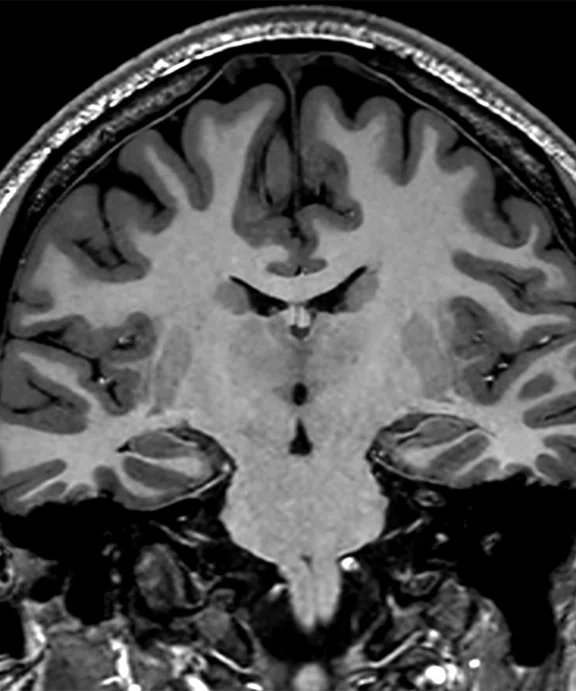
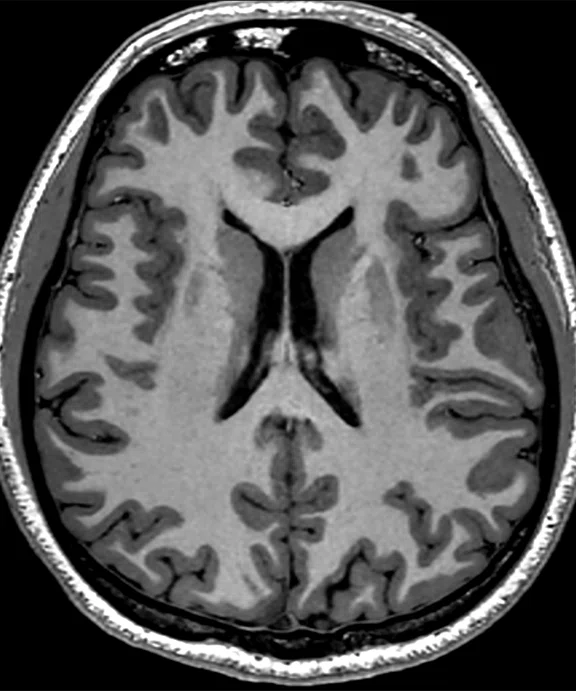
Figure 2.
(A) MP-RAGE sagittal with (B) coronal and (C) axial multiplanar reformats with AIR™ Recon DL, 0.7 x 0.7 x 0.7 mm, 5:31 min acquired on MAGNUS.
B
Figure 2.
(A) MP-RAGE sagittal with (B) coronal and (C) axial multiplanar reformats with AIR™ Recon DL, 0.7 x 0.7 x 0.7 mm, 5:31 min acquired on MAGNUS.
C
Figure 2.
(A) MP-RAGE sagittal with (B) coronal and (C) axial multiplanar reformats with AIR™ Recon DL, 0.7 x 0.7 x 0.7 mm, 5:31 min acquired on MAGNUS.
‡Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
result
PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
contact us • privacy policy • terms & conditions
© 2023 GE HealthCare. GE is a trademark of the General Electric Company used under trademark license.


Tech Trends
Pushing the envelope in neuroscience research with MAGNUS 3.0T
Pushing the envelope in neuroscience research with MAGNUS 3.0T
In mid-2023, the University of Iowa installed GE HealthCare’s investigational MAGNUS‡ 3.0T head-only MR system, an advanced gradient MR platform designed to deliver faster and stronger diffusion-weighted imaging while reducing the constraints of peripheral nerve stimulation. MAGNUS (Mesoscale diffusion with Advanced Gradients for Neuro Ultrafast Scanning) is a head-only MR scanner that complements the University of Iowa’s existing SIGNA™ 7.0T and SIGNA™ Premier 3.0T systems in its MR Research Facility.
MAGNUS could enable new research avenues in the study of psychiatric and neurological disorders, according to Vincent Magnotta, PhD, Director of the MR Research Facility and Professor of Radiology – Division of Neuroradiology.
“The high-performance gradients on MAGNUS will open up new opportunities at 3.0T, especially in very high b-value diffusion imaging,” Dr. Magnotta says. “While we are limited to b-values of around 3,000 at 3.0T and maybe 5,000 at 7.0T, with MAGNUS we can go up to b-values of 30,000 and beyond.”
The difference is in the ability to tease apart the signal coming from different tissue compartments, adds Merry Mani, PhD, Director of the Microstructure Imaging Lab, Radiology, and an Associate Professor of Radiology – Division of Neuroradiology.
“At higher b-values, we can obtain a much better resolution to see a wide range of the diffusivity and compartment sizes, making the imaging much more specific,” Dr. Mani says. “With this capability, we can look for biomarkers or other changes in response to a disease mechanism that cannot be detected at lower b-values.”
For example, they can study changes in the brain’s white matter in neurodegenerative diseases or early brain development in neonates and adolescents. So far, three ongoing studies – on multiple sclerosis (MS), bipolar disorder and autism spectrum disorders – have been migrated from the SIGNA™ Premier to MAGNUS. While the goal is to move more studies to MAGNUS, Dr. Mani is still in the process of refining and optimizing imaging protocols and evaluating the system’s advanced capabilities.
“We have the ability to push the spatial resolution to higher levels than with our other systems, so we need to test and evaluate the impact of this while at the same time understanding the full capabilities of MAGNUS,” adds Dr. Mani. “When we scanned at a b-value of 30,000 we could still get signal, which is something we can’t do with a human whole-body scanner with low gradient strengths.”
The team also scanned a donated brain fixed in a container on MAGNUS and were able to visualize tissue and structures. According to Dr. Mani, if they attempted diffusion scans on other MR systems, there would be too much noise to discern these type of details. Postmortem imaging is often utilized to obtain brain connectivity at ultra-high-resolution as well as help validate many in vivo modeling approaches.
“We’ve also been surprised at how good the anatomical imaging is on MAGNUS,” adds Dr. Magnotta. “We thought it would be similar to our other 3.0T system, however, with reduced echo spacing we can clearly see less blurring in the reconstructed images. It’s one of those surprises that is very pleasing to see.”
With the shorter TE from reduced echo spacing, there is higher SNR, which can lead to shorter TR periods for certain sequences and even higher b-values than what has so far been achieved. With RF transmit in a head-only configuration, Dr. Magnotta says it may be possible to image patient populations with MR-Conditional implanted devices, such as electrodes/deep brain stimulators and cardiac pacemakers, that are active outside the imaging FOV.
For facilities dedicated to neuroimaging, the potential utilization of a head-only MR system could help address anticipated volume growth, such as the need to monitor Alzheimer’s disease (AD) patients who are receiving amyloid-targeting treatments for amyloid-related imaging abnormalities (ARIA). More importantly, MAGNUS could help propel biomarker research in early-onset AD patients and potentially help provide a more definitive, earlier diagnosis when treatments may be more effective in slowing disease progression.
“Diffusion MR is a great tool to study all types of neurodegenerative diseases, and having a scanner that allows us to study them in greater detail may provide the data needed to find new biomarkers,” Dr. Mani adds.
For example, Dr. Mani envisions utilizing MAGNUS for effective axonal diameter mapping in vivo and to differentiate changes in the axonal compartment from the extra-axonal compartment. “With higher resolution imaging, we can get better differentiation of the compartments,” she says. “Nearly all of the diffusion-based biomarkers that were developed with lower resolutions will definitely get better and more specific with higher resolution, especially with the short TE made possible by the MAGNUS gradients.” In addition, Dr. Mani expects MAGNUS may provide better characterization of neuroinflammation in MS patients.
Figure 1.
Comparison of single-shot diffusion weighted images with b3000 and AIR™ Recon DL on (A) SIGNA™ Premier 3.0T using a GE HealthCare 48-ch head coil and (B) MAGNUS 3.0T using a Nova Medical 32-ch coil. Both data sets were acquired with the same parameters except TE, which was 71 ms for SIGNA™ Premier and 36 ms for MAGNUS. Note the higher SNR and reduced distortion in the MAGNUS image. (C) Diffusion tensor b30,000, TE=55 msec. with AIR™ Recon DL acquired on MAGNUS.
Other pre-clinical studies, such as time-dependent diffusivity or oscillating gradient diffusion, may be translated to in vivo studies thanks to the gradient power of MAGNUS. There are new areas of MR research that may now be possible with MAGNUS, such as multidimensional diffusion, micro-anisotropy or better characterization of the tissue microenvironment.
“Clearly, MAGNUS has a role in neuro imaging, whether it is the tissue microstructure, high b-value diffusion imaging or even anatomical imaging,” says Dr. Magnotta. MAGNUS can further complement the spectroscopy and susceptibility-weighed imaging that the University of Iowa conducts on its SIGNA™ 7.0T system. And, although SIGNA™ Premier is a high-end, whole-body scanner, the higher gradient performance of MAGNUS will allow the center to perform imaging studies that were not previously possible at 3.0T.

Read more about MAGNUS at:
https://ge-signapulse.readz.com/the-magnus-gradient-platform-advancing-the-f-7ywwm
DOWNLOAD ARTICLE HERE