result
A
Figure 1.
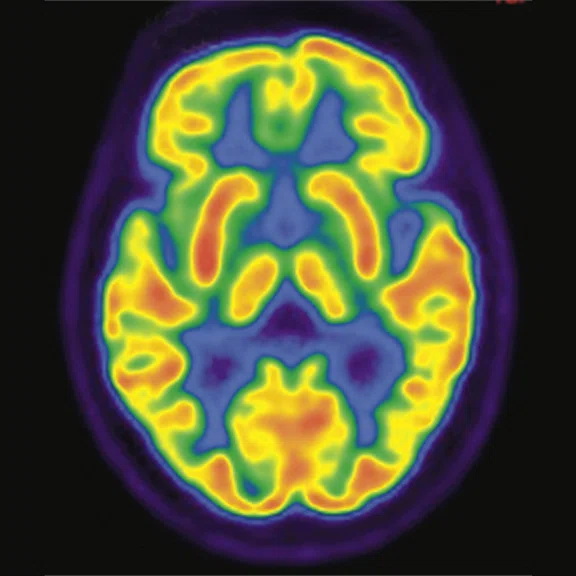
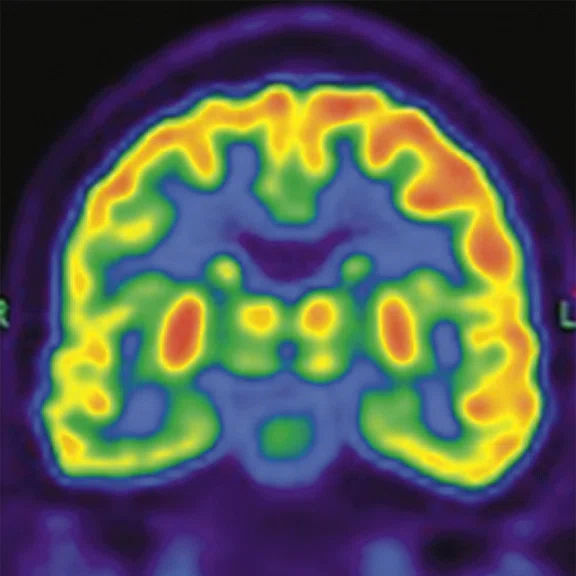
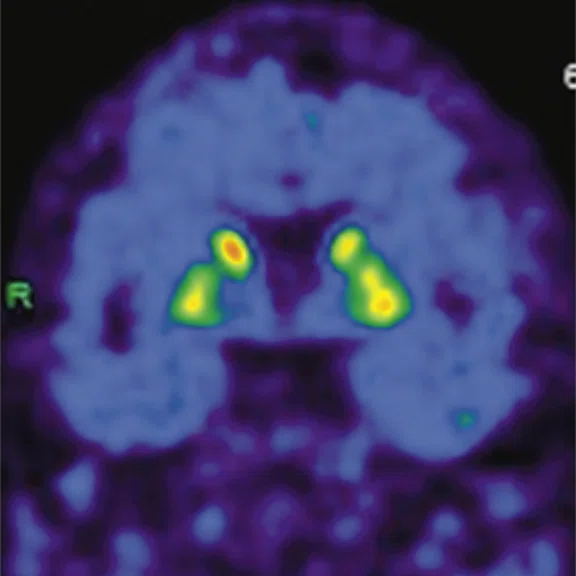
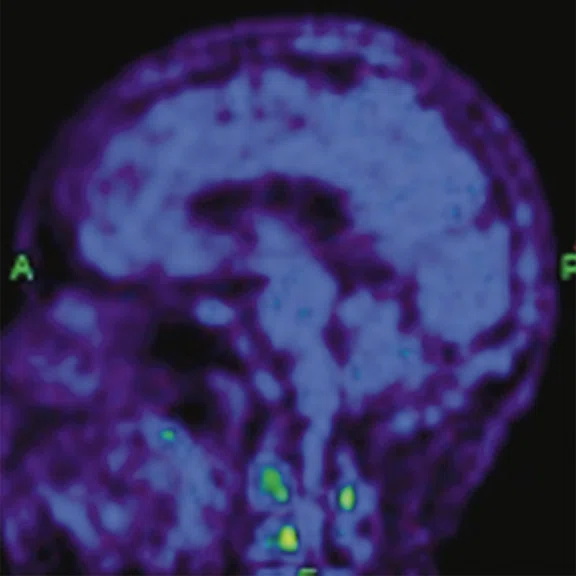
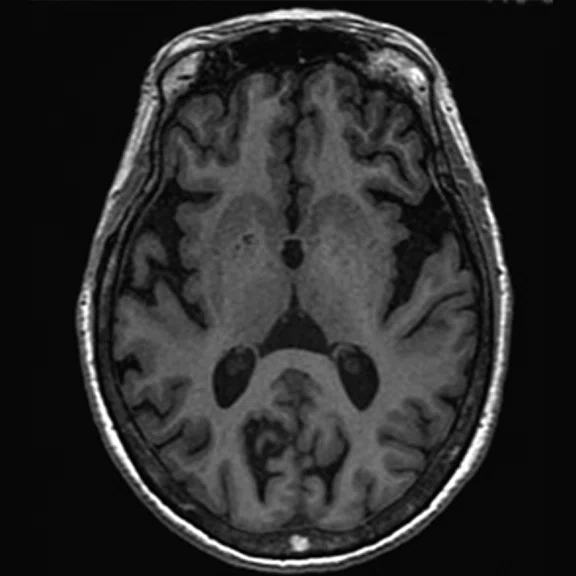
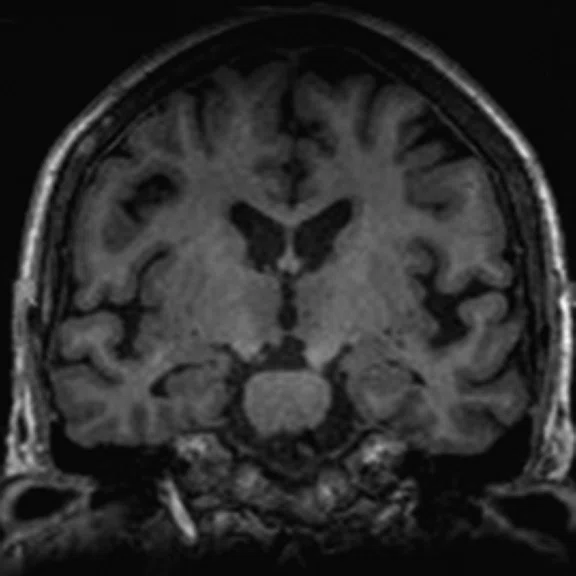
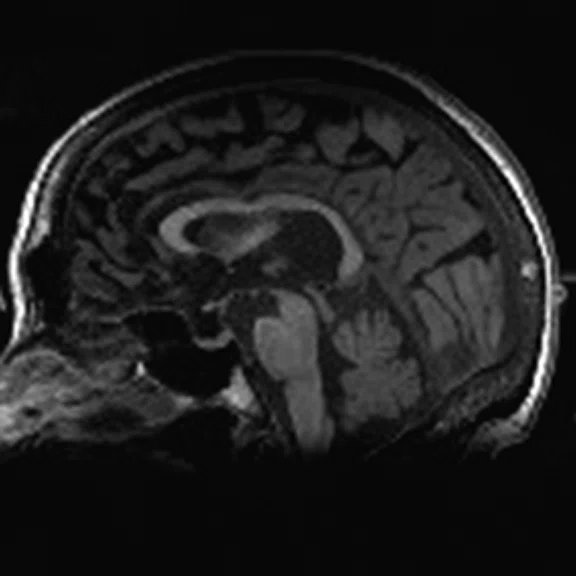
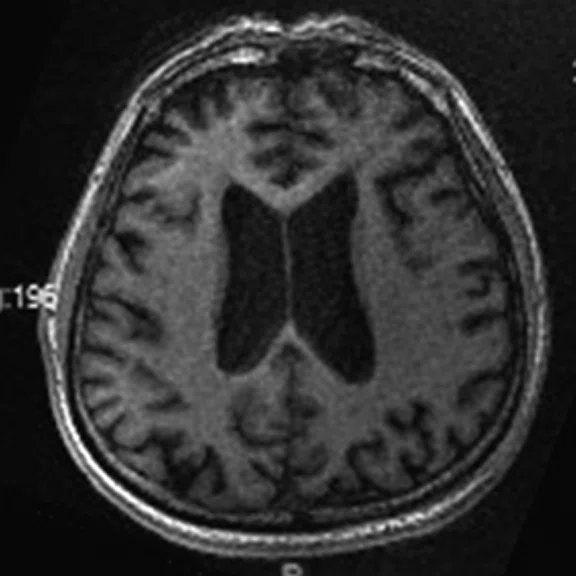
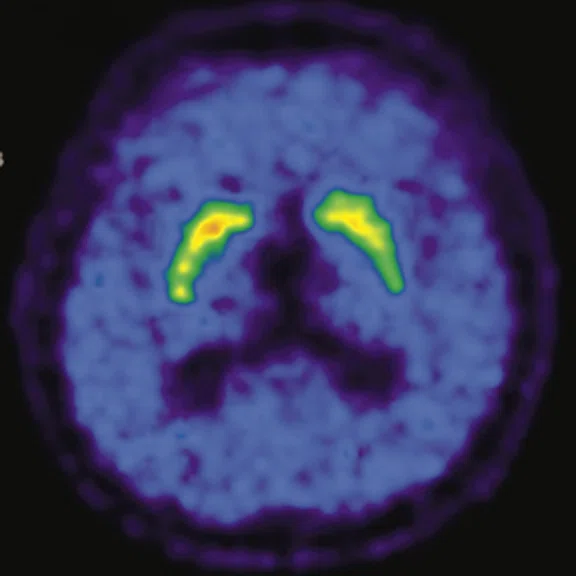
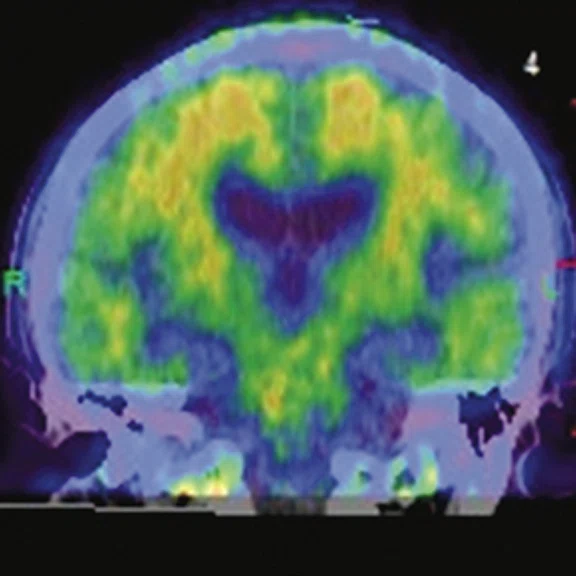
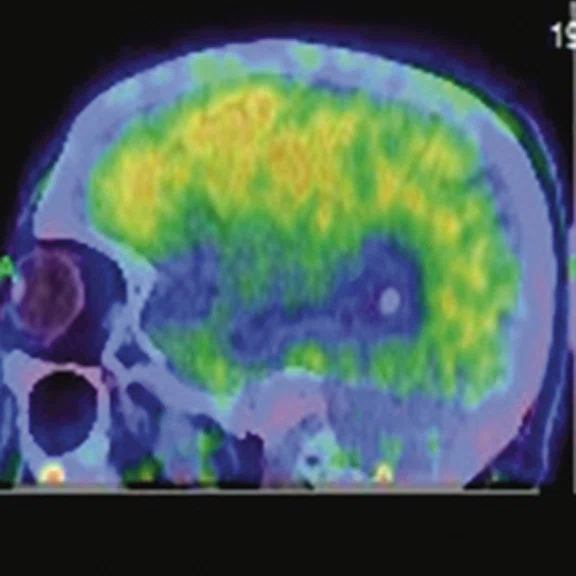
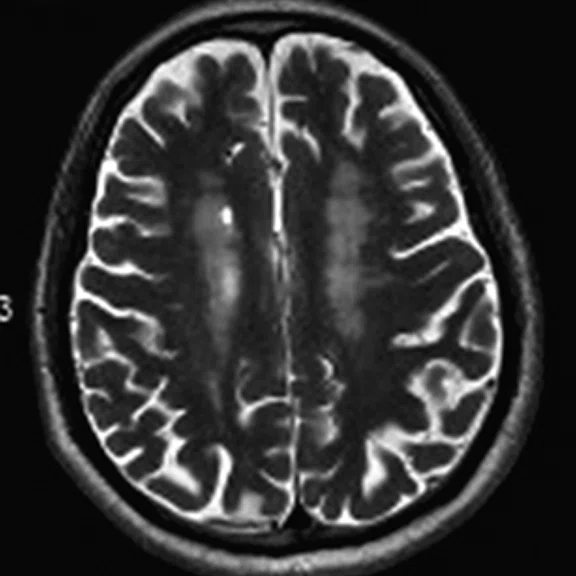
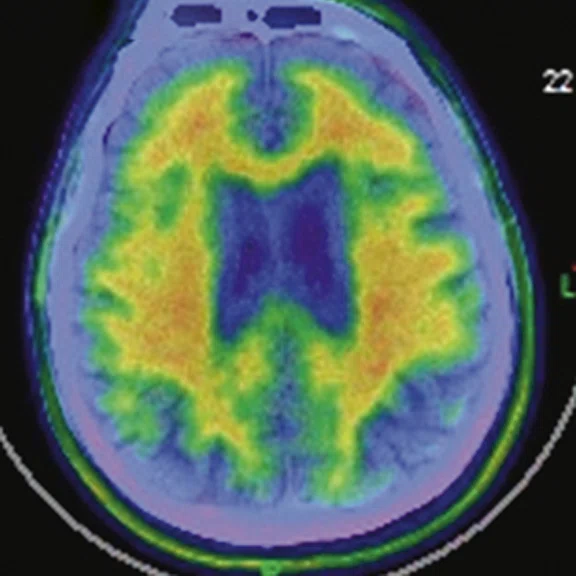
A 66-year-old female presented with weakness and tremor of the left lower limb with bradykinesia for more than four years. Levodopa test: the improvement rate was 30.56% in 30 min, 47.22% in 1 hour and 52.78% in 2 hours. Olfactory test indicated hyposmia. The symmetry of glucose metabolism in bilateral basal ganglia and thalamus increased, but glucose metabolism in bilateral cerebral cortex and cerebellum was not significantly decreased. The function of dopamine transporter (DAT) in the posterior part of the bilateral putamen was severely decreased, and the function of DAT in the anterior part of the bilateral putamen was slightly to moderately decreased, especially in the right side. From the clinical and imaging features, the patient was diagnosed with Parkinson’s disease.
B
Figure 1.
A 66-year-old female presented with weakness and tremor of the left lower limb with bradykinesia for more than four years. Levodopa test: the improvement rate was 30.56% in 30 min, 47.22% in 1 hour and 52.78% in 2 hours. Olfactory test indicated hyposmia. The symmetry of glucose metabolism in bilateral basal ganglia and thalamus increased, but glucose metabolism in bilateral cerebral cortex and cerebellum was not significantly decreased. The function of dopamine transporter (DAT) in the posterior part of the bilateral putamen was severely decreased, and the function of DAT in the anterior part of the bilateral putamen was slightly to moderately decreased, especially in the right side. From the clinical and imaging features, the patient was diagnosed with Parkinson’s disease.
C
Figure 1.
A 66-year-old female presented with weakness and tremor of the left lower limb with bradykinesia for more than four years. Levodopa test: the improvement rate was 30.56% in 30 min, 47.22% in 1 hour and 52.78% in 2 hours. Olfactory test indicated hyposmia. The symmetry of glucose metabolism in bilateral basal ganglia and thalamus increased, but glucose metabolism in bilateral cerebral cortex and cerebellum was not significantly decreased. The function of dopamine transporter (DAT) in the posterior part of the bilateral putamen was severely decreased, and the function of DAT in the anterior part of the bilateral putamen was slightly to moderately decreased, especially in the right side. From the clinical and imaging features, the patient was diagnosed with Parkinson’s disease.
D
Figure 1.
A 66-year-old female presented with weakness and tremor of the left lower limb with bradykinesia for more than four years. Levodopa test: the improvement rate was 30.56% in 30 min, 47.22% in 1 hour and 52.78% in 2 hours. Olfactory test indicated hyposmia. The symmetry of glucose metabolism in bilateral basal ganglia and thalamus increased, but glucose metabolism in bilateral cerebral cortex and cerebellum was not significantly decreased. The function of dopamine transporter (DAT) in the posterior part of the bilateral putamen was severely decreased, and the function of DAT in the anterior part of the bilateral putamen was slightly to moderately decreased, especially in the right side. From the clinical and imaging features, the patient was diagnosed with Parkinson’s disease.
E
Figure 1.
A 66-year-old female presented with weakness and tremor of the left lower limb with bradykinesia for more than four years. Levodopa test: the improvement rate was 30.56% in 30 min, 47.22% in 1 hour and 52.78% in 2 hours. Olfactory test indicated hyposmia. The symmetry of glucose metabolism in bilateral basal ganglia and thalamus increased, but glucose metabolism in bilateral cerebral cortex and cerebellum was not significantly decreased. The function of dopamine transporter (DAT) in the posterior part of the bilateral putamen was severely decreased, and the function of DAT in the anterior part of the bilateral putamen was slightly to moderately decreased, especially in the right side. From the clinical and imaging features, the patient was diagnosed with Parkinson’s disease.
F
Figure 1.
A 66-year-old female presented with weakness and tremor of the left lower limb with bradykinesia for more than four years. Levodopa test: the improvement rate was 30.56% in 30 min, 47.22% in 1 hour and 52.78% in 2 hours. Olfactory test indicated hyposmia. The symmetry of glucose metabolism in bilateral basal ganglia and thalamus increased, but glucose metabolism in bilateral cerebral cortex and cerebellum was not significantly decreased. The function of dopamine transporter (DAT) in the posterior part of the bilateral putamen was severely decreased, and the function of DAT in the anterior part of the bilateral putamen was slightly to moderately decreased, especially in the right side. From the clinical and imaging features, the patient was diagnosed with Parkinson’s disease.
G
Figure 1.
A 66-year-old female presented with weakness and tremor of the left lower limb with bradykinesia for more than four years. Levodopa test: the improvement rate was 30.56% in 30 min, 47.22% in 1 hour and 52.78% in 2 hours. Olfactory test indicated hyposmia. The symmetry of glucose metabolism in bilateral basal ganglia and thalamus increased, but glucose metabolism in bilateral cerebral cortex and cerebellum was not significantly decreased. The function of dopamine transporter (DAT) in the posterior part of the bilateral putamen was severely decreased, and the function of DAT in the anterior part of the bilateral putamen was slightly to moderately decreased, especially in the right side. From the clinical and imaging features, the patient was diagnosed with Parkinson’s disease.
H
Figure 1.
A 66-year-old female presented with weakness and tremor of the left lower limb with bradykinesia for more than four years. Levodopa test: the improvement rate was 30.56% in 30 min, 47.22% in 1 hour and 52.78% in 2 hours. Olfactory test indicated hyposmia. The symmetry of glucose metabolism in bilateral basal ganglia and thalamus increased, but glucose metabolism in bilateral cerebral cortex and cerebellum was not significantly decreased. The function of dopamine transporter (DAT) in the posterior part of the bilateral putamen was severely decreased, and the function of DAT in the anterior part of the bilateral putamen was slightly to moderately decreased, especially in the right side. From the clinical and imaging features, the patient was diagnosed with Parkinson’s disease.
I
Figure 1.
A 66-year-old female presented with weakness and tremor of the left lower limb with bradykinesia for more than four years. Levodopa test: the improvement rate was 30.56% in 30 min, 47.22% in 1 hour and 52.78% in 2 hours. Olfactory test indicated hyposmia. The symmetry of glucose metabolism in bilateral basal ganglia and thalamus increased, but glucose metabolism in bilateral cerebral cortex and cerebellum was not significantly decreased. The function of dopamine transporter (DAT) in the posterior part of the bilateral putamen was severely decreased, and the function of DAT in the anterior part of the bilateral putamen was slightly to moderately decreased, especially in the right side. From the clinical and imaging features, the patient was diagnosed with Parkinson’s disease.
A
Figure 2.
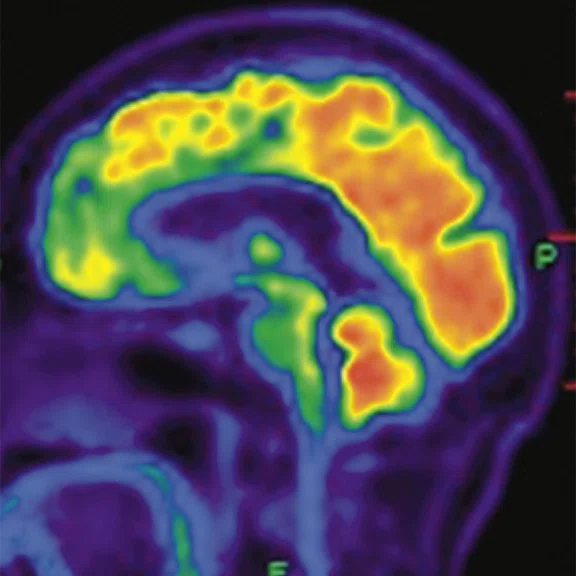
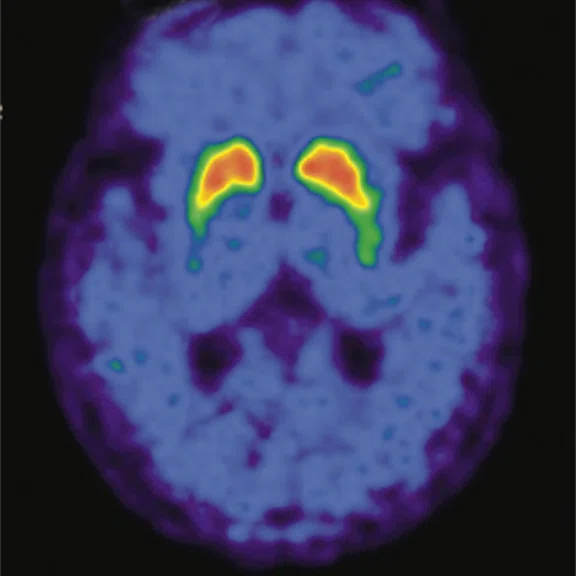
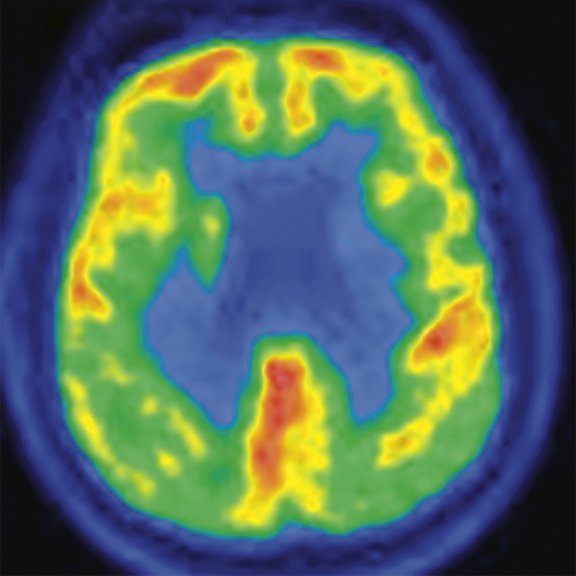
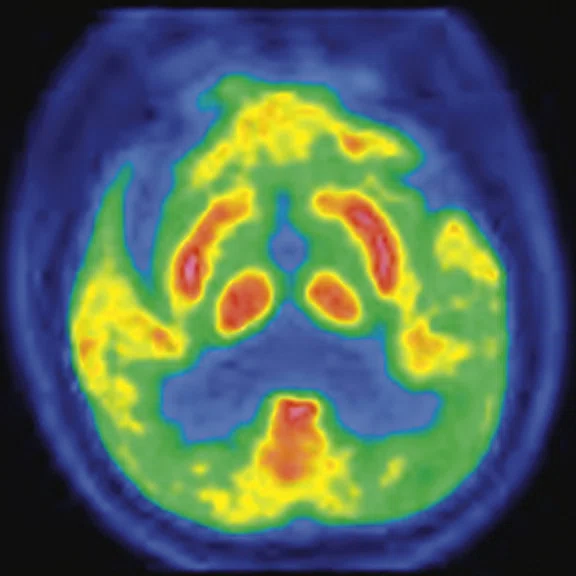
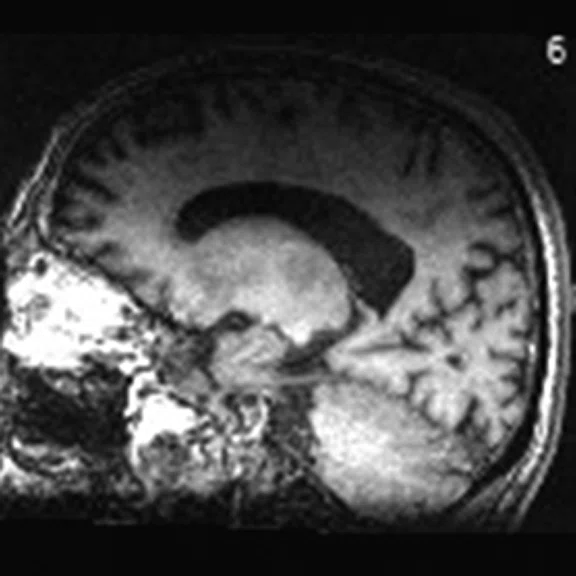
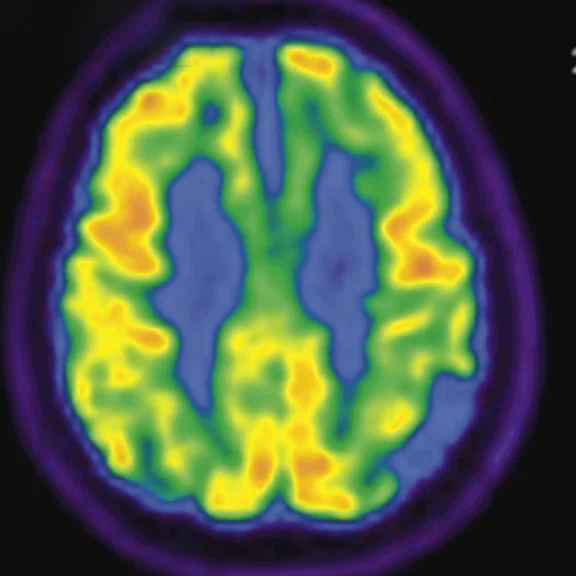
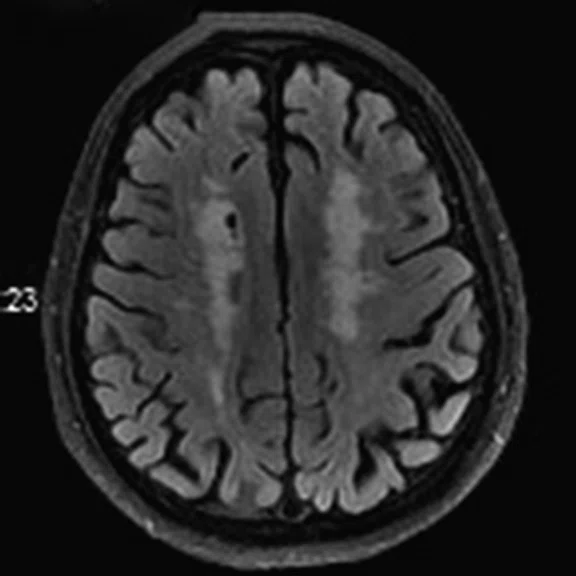
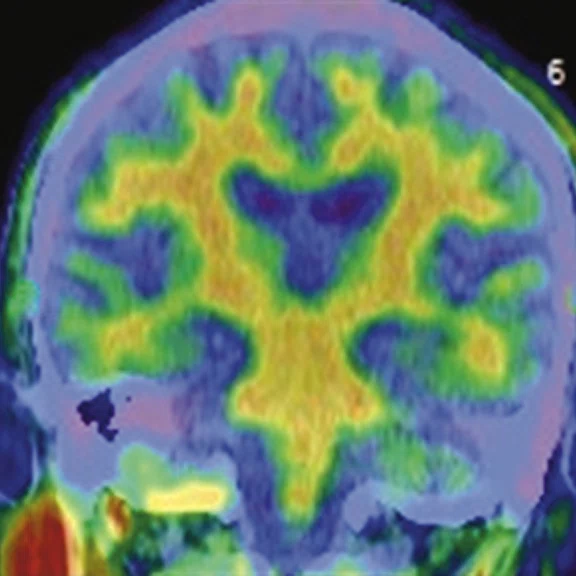
A 76-year-old male presented with memory decline and weakness of lower limbs for two years. MMSE score: 14, MOCA score: 11. The glucose metabolism of bilateral parietal lobes is decreased, which seems to be “posterior cingulate Island sign” (red arrow). The function of dopamine transporter in the middle and posterior putamen decreased. Deposition of amyloid plaques in bilateral frontal parietal cortex and posterior cingulate gyrus. From the clinical and imaging features, the patient was diagnosed with Alzheimer’s disease (AD) and dementia with Lewy body (DLB).
B
Figure 2.
A 76-year-old male presented with memory decline and weakness of lower limbs for two years. MMSE score: 14, MOCA score: 11. The glucose metabolism of bilateral parietal lobes is decreased, which seems to be “posterior cingulate Island sign” (red arrow). The function of dopamine transporter in the middle and posterior putamen decreased. Deposition of amyloid plaques in bilateral frontal parietal cortex and posterior cingulate gyrus. From the clinical and imaging features, the patient was diagnosed with Alzheimer’s disease (AD) and dementia with Lewy body (DLB).
C
Figure 2.
A 76-year-old male presented with memory decline and weakness of lower limbs for two years. MMSE score: 14, MOCA score: 11. The glucose metabolism of bilateral parietal lobes is decreased, which seems to be “posterior cingulate Island sign” (red arrow). The function of dopamine transporter in the middle and posterior putamen decreased. Deposition of amyloid plaques in bilateral frontal parietal cortex and posterior cingulate gyrus. From the clinical and imaging features, the patient was diagnosed with Alzheimer’s disease (AD) and dementia with Lewy body (DLB).
D
Figure 2.
A 76-year-old male presented with memory decline and weakness of lower limbs for two years. MMSE score: 14, MOCA score: 11. The glucose metabolism of bilateral parietal lobes is decreased, which seems to be “posterior cingulate Island sign” (red arrow). The function of dopamine transporter in the middle and posterior putamen decreased. Deposition of amyloid plaques in bilateral frontal parietal cortex and posterior cingulate gyrus. From the clinical and imaging features, the patient was diagnosed with Alzheimer’s disease (AD) and dementia with Lewy body (DLB).
E
Figure 2.
A 76-year-old male presented with memory decline and weakness of lower limbs for two years. MMSE score: 14, MOCA score: 11. The glucose metabolism of bilateral parietal lobes is decreased, which seems to be “posterior cingulate Island sign” (red arrow). The function of dopamine transporter in the middle and posterior putamen decreased. Deposition of amyloid plaques in bilateral frontal parietal cortex and posterior cingulate gyrus. From the clinical and imaging features, the patient was diagnosed with Alzheimer’s disease (AD) and dementia with Lewy body (DLB).
F
Figure 2.
A 76-year-old male presented with memory decline and weakness of lower limbs for two years. MMSE score: 14, MOCA score: 11. The glucose metabolism of bilateral parietal lobes is decreased, which seems to be “posterior cingulate Island sign” (red arrow). The function of dopamine transporter in the middle and posterior putamen decreased. Deposition of amyloid plaques in bilateral frontal parietal cortex and posterior cingulate gyrus. From the clinical and imaging features, the patient was diagnosed with Alzheimer’s disease (AD) and dementia with Lewy body (DLB).
G
Figure 2.
A 76-year-old male presented with memory decline and weakness of lower limbs for two years. MMSE score: 14, MOCA score: 11. The glucose metabolism of bilateral parietal lobes is decreased, which seems to be “posterior cingulate Island sign” (red arrow). The function of dopamine transporter in the middle and posterior putamen decreased. Deposition of amyloid plaques in bilateral frontal parietal cortex and posterior cingulate gyrus. From the clinical and imaging features, the patient was diagnosed with Alzheimer’s disease (AD) and dementia with Lewy body (DLB).
H
Figure 2.
A 76-year-old male presented with memory decline and weakness of lower limbs for two years. MMSE score: 14, MOCA score: 11. The glucose metabolism of bilateral parietal lobes is decreased, which seems to be “posterior cingulate Island sign” (red arrow). The function of dopamine transporter in the middle and posterior putamen decreased. Deposition of amyloid plaques in bilateral frontal parietal cortex and posterior cingulate gyrus. From the clinical and imaging features, the patient was diagnosed with Alzheimer’s disease (AD) and dementia with Lewy body (DLB).
I
Figure 2.
A 76-year-old male presented with memory decline and weakness of lower limbs for two years. MMSE score: 14, MOCA score: 11. The glucose metabolism of bilateral parietal lobes is decreased, which seems to be “posterior cingulate Island sign” (red arrow). The function of dopamine transporter in the middle and posterior putamen decreased. Deposition of amyloid plaques in bilateral frontal parietal cortex and posterior cingulate gyrus. From the clinical and imaging features, the patient was diagnosed with Alzheimer’s disease (AD) and dementia with Lewy body (DLB).
A
Figure 3.
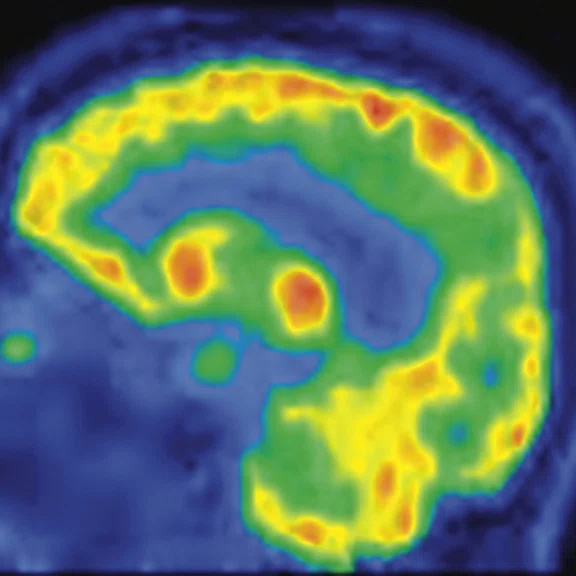
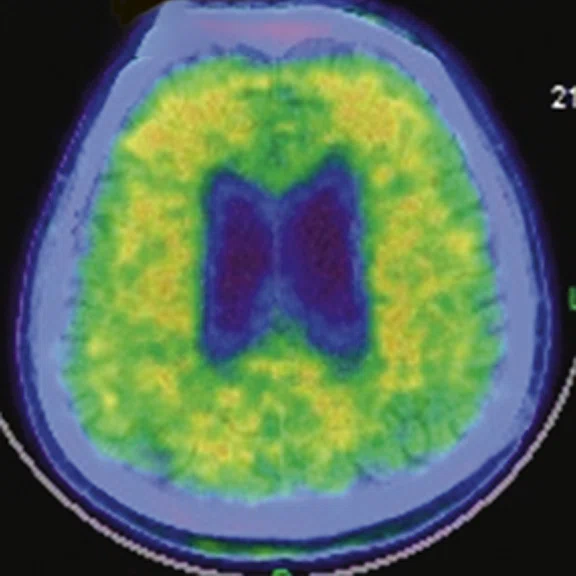
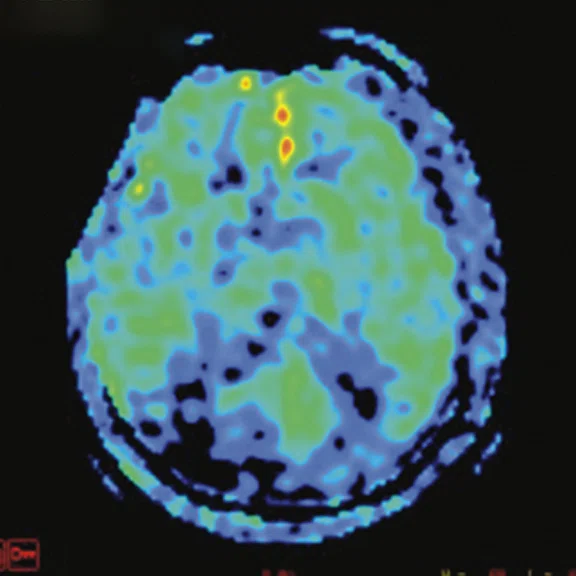
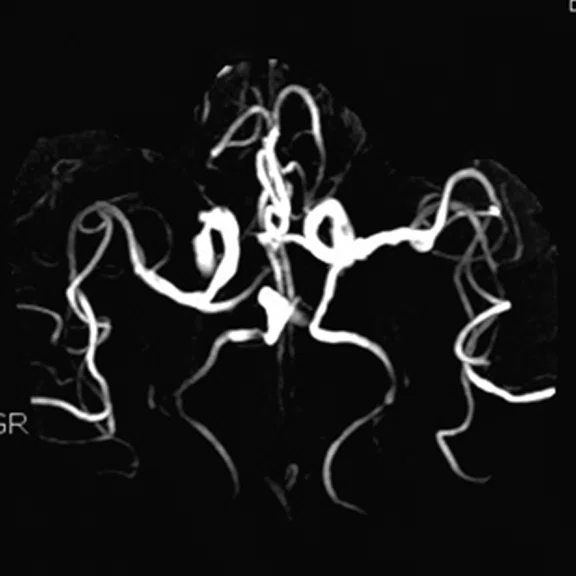
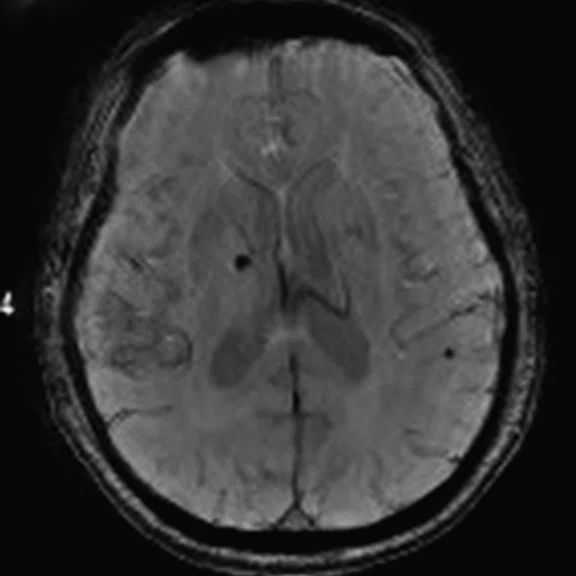
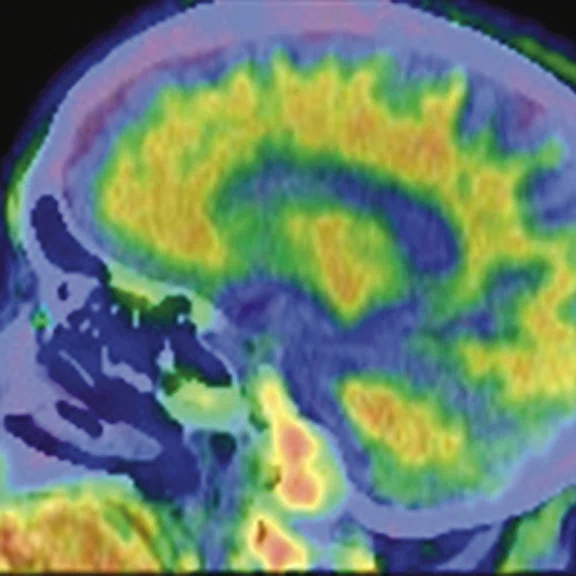
A 79-year-old male with memory decline and bradykinesia for more than one year. MMSE score: 20. MOCA score: 13. The glucose metabolism of bilateral frontal, parietal and temporal lobes decreased unevenly. Mild deposition of amyloid plaques in subcortical area. Also diagnosed with brain atrophy; leukoaraiosis; multiple lumen infarcts; multiple microbleeds and atherosclerosis of intracranial artery and bilateral internal carotid artery. From the clinical and imaging features, the patient was diagnosed with vascular dementia.
B
Figure 3.
A 79-year-old male with memory decline and bradykinesia for more than one year. MMSE score: 20. MOCA score: 13. The glucose metabolism of bilateral frontal, parietal and temporal lobes decreased unevenly. Mild deposition of amyloid plaques in subcortical area. Also diagnosed with brain atrophy; leukoaraiosis; multiple lumen infarcts; multiple microbleeds and atherosclerosis of intracranial artery and bilateral internal carotid artery. From the clinical and imaging features, the patient was diagnosed with vascular dementia.
C
Figure 3.
A 79-year-old male with memory decline and bradykinesia for more than one year. MMSE score: 20. MOCA score: 13. The glucose metabolism of bilateral frontal, parietal and temporal lobes decreased unevenly. Mild deposition of amyloid plaques in subcortical area. Also diagnosed with brain atrophy; leukoaraiosis; multiple lumen infarcts; multiple microbleeds and atherosclerosis of intracranial artery and bilateral internal carotid artery. From the clinical and imaging features, the patient was diagnosed with vascular dementia.
D
Figure 3.
A 79-year-old male with memory decline and bradykinesia for more than one year. MMSE score: 20. MOCA score: 13. The glucose metabolism of bilateral frontal, parietal and temporal lobes decreased unevenly. Mild deposition of amyloid plaques in subcortical area. Also diagnosed with brain atrophy; leukoaraiosis; multiple lumen infarcts; multiple microbleeds and atherosclerosis of intracranial artery and bilateral internal carotid artery. From the clinical and imaging features, the patient was diagnosed with vascular dementia.
E
Figure 3.
A 79-year-old male with memory decline and bradykinesia for more than one year. MMSE score: 20. MOCA score: 13. The glucose metabolism of bilateral frontal, parietal and temporal lobes decreased unevenly. Mild deposition of amyloid plaques in subcortical area. Also diagnosed with brain atrophy; leukoaraiosis; multiple lumen infarcts; multiple microbleeds and atherosclerosis of intracranial artery and bilateral internal carotid artery. From the clinical and imaging features, the patient was diagnosed with vascular dementia.
F
Figure 3.
A 79-year-old male with memory decline and bradykinesia for more than one year. MMSE score: 20. MOCA score: 13. The glucose metabolism of bilateral frontal, parietal and temporal lobes decreased unevenly. Mild deposition of amyloid plaques in subcortical area. Also diagnosed with brain atrophy; leukoaraiosis; multiple lumen infarcts; multiple microbleeds and atherosclerosis of intracranial artery and bilateral internal carotid artery. From the clinical and imaging features, the patient was diagnosed with vascular dementia.
G
Figure 3.
A 79-year-old male with memory decline and bradykinesia for more than one year. MMSE score: 20. MOCA score: 13. The glucose metabolism of bilateral frontal, parietal and temporal lobes decreased unevenly. Mild deposition of amyloid plaques in subcortical area. Also diagnosed with brain atrophy; leukoaraiosis; multiple lumen infarcts; multiple microbleeds and atherosclerosis of intracranial artery and bilateral internal carotid artery. From the clinical and imaging features, the patient was diagnosed with vascular dementia.
H
Figure 3.
A 79-year-old male with memory decline and bradykinesia for more than one year. MMSE score: 20. MOCA score: 13. The glucose metabolism of bilateral frontal, parietal and temporal lobes decreased unevenly. Mild deposition of amyloid plaques in subcortical area. Also diagnosed with brain atrophy; leukoaraiosis; multiple lumen infarcts; multiple microbleeds and atherosclerosis of intracranial artery and bilateral internal carotid artery. From the clinical and imaging features, the patient was diagnosed with vascular dementia.
I
Figure 3.
A 79-year-old male with memory decline and bradykinesia for more than one year. MMSE score: 20. MOCA score: 13. The glucose metabolism of bilateral frontal, parietal and temporal lobes decreased unevenly. Mild deposition of amyloid plaques in subcortical area. Also diagnosed with brain atrophy; leukoaraiosis; multiple lumen infarcts; multiple microbleeds and atherosclerosis of intracranial artery and bilateral internal carotid artery. From the clinical and imaging features, the patient was diagnosed with vascular dementia.


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
IN PRACTICE
PET/MR delivers precision for differential diagnoses in neuroimaging
PET/MR delivers precision for differential diagnoses in neuroimaging
As one of the first nuclear medicine departments in China dating to 1958, Wuhan Union Hospital boasts an impressive array of imaging systems and radiotracer development. These include two PET/CT systems, three SPECT/ CT systems, animal PET/CT and SPECT/CT, two cyclotrons and, since 2017, SIGNA™ PET/MR. Nearly 4,000 patients have been examined on the SIGNA™ PET/MR by year-end 2020, with approximately 80% for clinical use and the remaining for research purposes only. Almost 60% (2,280) of patient studies are for neurological disease – oncology, neurodegenerative and epilepsy.
As one of the first nuclear medicine departments in China dating to 1958, Wuhan Union Hospital boasts an impressive array of imaging systems and radiotracer development. These include two PET/CT systems, three SPECT/ CT systems, animal PET/CT and SPECT/CT, two cyclotrons and, since 2017, SIGNA™ PET/MR. Nearly 4,000 patients have been examined on the SIGNA™ PET/MR by year-end 2020, with approximately 80% for clinical use and the remaining for research purposes only. Almost 60% (2,280) of patient studies are for neurological disease – oncology, neurodegenerative and epilepsy.
Wuhan Union Hospital also holds the nation’s highest level certificate for the development and use of radioactive tracers, making it a national leader in theranostics. The nuclear medicine department has a radionuclide therapy unit with 28 patient beds and is actively involved in researching the use of new tracers for diagnosis and therapy. During the initial COVID-19 pandemic in early 2020, the hospital paused nuclear imaging exams as the city went into lockdown. PET exams on oncology patients resumed slowly in March to avoid interruptions to their treatment as much as possible. By July 2020, the nuclear medicine department was able to resume pre-pandemic imaging volumes. Patients were tested for the virus, and if they were positive, excluded from a PET/MR or PET/CT exam until they recovered.
Professor Xiaoli Lan, MD, PhD, chief physician, chairperson of the department of nuclear medicine and PET Center, Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, believes that PET/MR should have an important role in the diagnosis and management of neurodegenerative diseases.
“There are different types of neurodegenerative diseases that are classified as Parkinson’s syndrome, or Parkinsonism, and Alzheimer’s disease,” Professor Lan explains. “Most important in the management of these diseases is to achieve a precise and differential diagnosis for each patient’s treatment decisions.”
For example, Parkinsonism includes multiple system atrophy (MSA), progressive supranuclear palsy (PSP), corticobasal syndrome (CBS), dementia with Lewy bodies, vascular Parkinsonism and drug-induced Parkinsonism. Alzheimer’s disease (AD) is a classification within dementia that includes three forms: late onset, early onset and familial. There is also frontotemporal dementia, a group of disorders that occurs when nerve cells in the frontal and temporal lobes of the brain are lost, causing the lobes to shrink.
The impressive array of radioactive tracers at Wuhan Union Hospital – more than 10 different radiotracers are produced in-house – enable precision in managing neurodegenerative disease patients at the hospital. According to Professor Lan, Parkinsonism can be successfully differentiated by utilizing glucose metabolic or dopamine transporter (11C-CFT) imaging in combination with MR anatomical structural imaging. AD patients are recommended for glucose metabolic, amyloid (11C-PIB or 18F-AV45) or tau protein (18F-APN1607) imaging. Each neurodegenerative disease patient at Wuhan Union undergoes at least one PET/MR exam to obtain the metabolic and anatomical imaging data.
“Combining PET and MR together, so the patient can undergo the imaging at the same time, is best,” Professor Lan explains. For example, if the PET detects differences in the tau protein (tau “tangles”) or amyloid deposits in regions of the brain, that information can be analyzed along with MR diffusion, perfusion or functional imaging.
“With SIGNA™ PET/MR, we are able to elevate our research on nervous system diseases such as epilepsy, Parkinson’s disease and Alzheimer’s disease. The application of PET molecular imaging probes such as 68Ga-DOTA-TATE in neuroendocrine tumors and 68Ga-PSMA in prostate cancer are exciting areas that we believe will further advance our research efforts,” Professor Lan says. “These are the diseases, along with pediatric cancer cases, where we will see the greatest benefit for improving patient care with PET/MR.”
Epilepsy and pediatric imaging
All patients with epilepsy undergo PET/ MR imaging at Wuhan Union Hospital. PET/MR can show the abnormal glucose metabolism with PET and also depict the abnormal brain structure with MR imaging, which is more conducive to the display of epileptic foci and the diagnosis of epilepsy etiology.
“We can diagnose epilepsy with PET/ MR,” says Professor Lan. “We can determine the location of the lesion, and that information can help us determine the cause of the epileptic seizures.”
Figure 1.
A 66-year-old female presented with weakness and tremor of the left lower limb with bradykinesia for more than four years. Levodopa test: the improvement rate was 30.56% in 30 min, 47.22% in 1 hour and 52.78% in 2 hours. Olfactory test indicated hyposmia. The symmetry of glucose metabolism in bilateral basal ganglia and thalamus increased, but glucose metabolism in bilateral cerebral cortex and cerebellum was not significantly decreased. The function of dopamine transporter (DAT) in the posterior part of the bilateral putamen was severely decreased, and the function of DAT in the anterior part of the bilateral putamen was slightly to moderately decreased, especially in the right side. From the clinical and imaging features, the patient was diagnosed with Parkinson’s disease.
For pediatric oncology patients, Professor Lan believes PET/MR should be utilized more often, especially in cases outside of the abdomen. Many oncology patients receive a whole-body PET/CT as well as a MR exam covering the region of interest.
“PET/MR can reduce the patient radiation exposure compared to PET/CT,” and that is particularly important in children with cancer, Professor Lan says. However, MR interpretation can be more challenging in abdominal exams due to the potential for artifacts from respiration and motion. For some children, it is more difficult to cooperate and remain still with the longer MR exam times. This is an area where advancements in MR imaging can decrease exam time, enable free breathing imaging and provide advanced image reconstruction techniques.
Oncologic imaging
PET/MR is routinely used in many oncology cases at Wuhan Union Hospital. All brain tumor patients routinely receive an 11C-methionine (11C-MET) PET/MR exam with an enhanced protocol. The hospital’s oncologic brain imaging includes different tumor types, such as nasopharyngeal carcinoma and head and neck tumors, as well as brain metastases from pancreatic, liver, cervical and other cancers.
All head and neck cancer patients receive whole-body PET/CT and head and neck PET/MR, with MR data acquired simultaneously with the PET acquisition. In cases of nasopharyngeal carcinoma, the FDG PET/MR data is used for radiotherapy planning, and an enhanced MR scan provides additional data for radiotherapy target planning.
Figure 2.
A 76-year-old male presented with memory decline and weakness of lower limbs for two years. MMSE score: 14, MOCA score: 11. The glucose metabolism of bilateral parietal lobes is decreased, which seems to be “posterior cingulate Island sign” (red arrow). The function of dopamine transporter in the middle and posterior putamen decreased. Deposition of amyloid plaques in bilateral frontal parietal cortex and posterior cingulate gyrus. From the clinical and imaging features, the patient was diagnosed with Alzheimer’s disease (AD) and dementia with Lewy body (DLB).
In some cases, PET/CT does not clearly depict abdominal and pelvic tumors, such as pancreatic and cervical cancer, Professor Lan explains. She suggests using PET/MR after the PET/CT exam to better visualize these lesions and their invasion into adjacent tissue.
PET/MR is also useful for diagnosing prostate cancer using the combination of 68Ga-PSMA with PET and multi-parametric MR imaging. However, Professor Lan adds that it’s clinical utility is best in stage I and stage II prostate cancers as well as non-metastatic disease.
Figure 3.
A 79-year-old male with memory decline and bradykinesia for more than one year. MMSE score: 20. MOCA score: 13. The glucose metabolism of bilateral frontal, parietal and temporal lobes decreased unevenly. Mild deposition of amyloid plaques in subcortical area. Also diagnosed with brain atrophy; leukoaraiosis; multiple lumen infarcts; multiple microbleeds and atherosclerosis of intracranial artery and bilateral internal carotid artery. From the clinical and imaging features, the patient was diagnosed with vascular dementia.
Looking forward, Professor Lan believes that PET/MR provides advantages in the diagnosis of brain diseases including epilepsy, brain tumors and neurodegenerative diseases. The continued development of novel tracers will continue to propel the use of PET/MR. However, she adds that PET/MR is a relatively new modality with only one decade in clinical use and, therefore, it is important to continue exploring key applications of PET/MR. She is hopeful that as more hospitals throughout China acquire a PET/MR system – the Chinese government has issued 50 licenses for new PET/MR systems in the next three years – multi-center studies and clinical trials can be initiated to further explore the value of this powerful imaging modality.