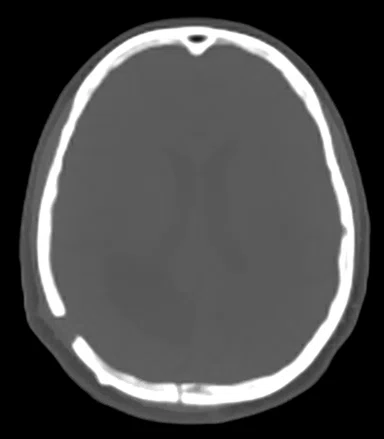
A
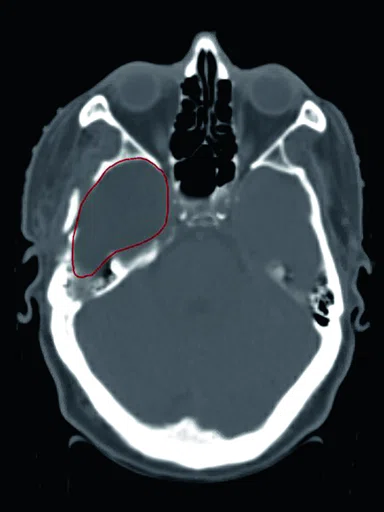
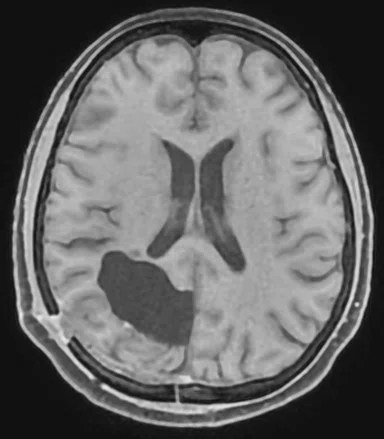
Figure 1.
Comparison of (A) CT and (B) sCT images for a representative case, showing excellent agreement in Hounsfield units (HU) and bone structures. The tumor is outlined in red.
B
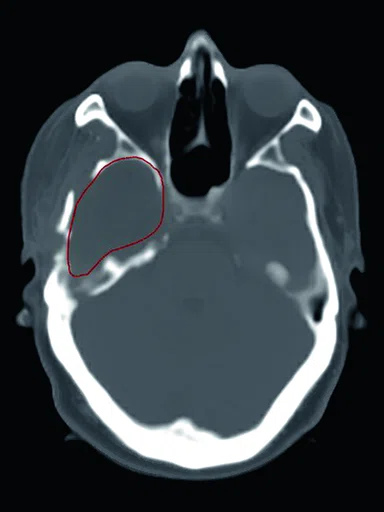
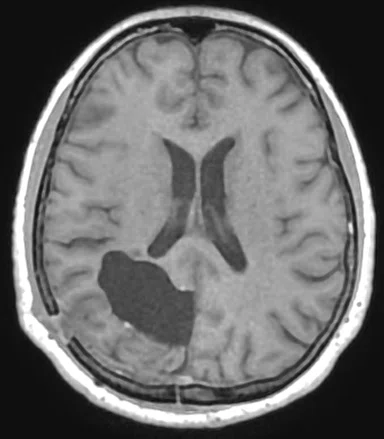
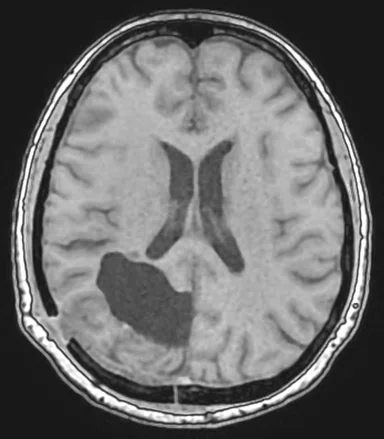
Figure 1.
Comparison of (A) CT and (B) sCT images for a representative case, showing excellent agreement in Hounsfield units (HU) and bone structures. The tumor is outlined in red.
A
Figure 3.
All four Dixon output images are used for sCT generation in the software MRI Planner; (A) water, (B) fat, (C) in phase and (D) out of phase. (E) The resulting sCT image is automatically returned to the treatment planning system.
B
Figure 3.
All four Dixon output images are used for sCT generation in the software MRI Planner; (A) water, (B) fat, (C) in phase and (D) out of phase. (E) The resulting sCT image is automatically returned to the treatment planning system.
C
Figure 3.
All four Dixon output images are used for sCT generation in the software MRI Planner; (A) water, (B) fat, (C) in phase and (D) out of phase. (E) The resulting sCT image is automatically returned to the treatment planning system.
D
Figure 3.
All four Dixon output images are used for sCT generation in the software MRI Planner; (A) water, (B) fat, (C) in phase and (D) out of phase. (E) The resulting sCT image is automatically returned to the treatment planning system.
E
Figure 3.
All four Dixon output images are used for sCT generation in the software MRI Planner; (A) water, (B) fat, (C) in phase and (D) out of phase. (E) The resulting sCT image is automatically returned to the treatment planning system.
F
Figure #.
Figure Copy.
1. Persson E, Jamtheim Gustafsson C, Ambolt P, et al. MR-PROTECT: Clinical feasibility of a prostate MRI-only radiotherapy treatment workflow and investigation of acceptance criteria. Radiat Oncol. 2020 Apr 9;15(1):77.
2. Lerner M, Medin J, Jamtheim Gustafsson C, et al. Clinical validation of a commercially available deep learning software for synthetic CT generation for brain. Radiat Oncol. 2021 Apr 7;16(1):66.
3. Lerner M, Medin J, Jamtheim Gustafsson C, Alkner S and Olsson LE. Prospective Clinical Feasibility Study for MRI-Only Brain Radiotherapy. Front Oncol. 2022 Jan 10;11:812643.
2. Lerner M, Medin J, Jamtheim Gustafsson C, et al. Clinical validation of a commercially available deep learning software for synthetic CT generation for brain. Radiat Oncol. 2021 Apr 7;16(1):66.
4. Paulson ES, Crijns SP, Keller BM, et al. Consensus opinion on MRI simulation for external beam radiation treatment planning. Radiother Oncol. 2016 Nov;121(2):187–92.
3. Lerner M, Medin J, Jamtheim Gustafsson C, Alkner S and Olsson LE. Prospective Clinical Feasibility Study for MRI-Only Brain Radiotherapy. Front Oncol. 2022 Jan 10;11:812643.
5. Ulin K, Urie MM, Cherlow JM. Results of a multi-institutional benchmark test for cranial CT/MR image registration. Int J Radiat Oncol Biol Phys. 2010 Aug 1;77(5):1584–9.
A
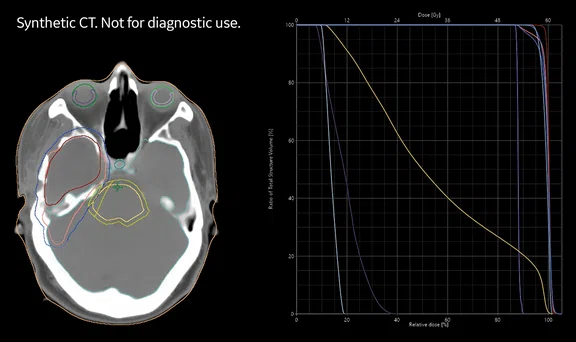
Figure 2.
(A) Dose calculations are based on sCT images in the treatment planning system. Evaluation of the dose volume histogram for treatment approval fullfilled the clinical acceptance criteria for all patients. (B) sCT with dose distribution displayed as color wash with a cut-off dose at 10% of the prescribed dose.
B
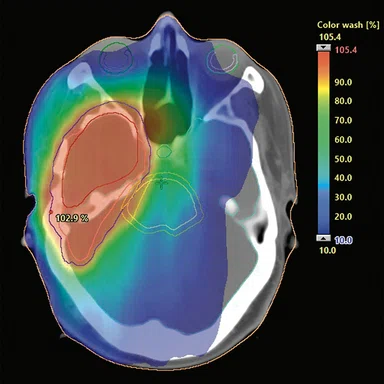
Figure 2.
(A) Dose calculations are based on sCT images in the treatment planning system. Evaluation of the dose volume histogram for treatment approval fullfilled the clinical acceptance criteria for all patients. (B) sCT with dose distribution displayed as color wash with a cut-off dose at 10% of the prescribed dose.


result
PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
IN PRACTICE
Successful implementation of an MR-only radiotherapy workflow
Successful implementation of an MR-only radiotherapy workflow
Since 2017, Skåne University Hospital has collaborated with other Swedish hospitals on the development of an MR-only workflow for radiotherapy. Funded by the Swedish innovation agency, Vinnova, and associated hospitals, the Gentle Radiotherapy program is creating a large-scale national world-class platform for cancer treatment using MR to describe tumor morphology instead of CT.
Since 2017, Skåne University Hospital has collaborated with other Swedish hospitals on the development of an MR-only workflow for radiotherapy. Funded by the Swedish innovation agency, Vinnova, and associated hospitals, the Gentle Radiotherapy program is creating a large-scale national world-class platform for cancer treatment using MR to describe tumor morphology instead of CT.
A key motivation behind this research is to improve the accuracy of treatment delivery, which then enables an escalation in radiation dose to cancer cells with more precision, thereby minimizing dose to surrounding healthy tissue and organs. The primary organ sites for Skåne University Hospital are the prostate and brain.
“The reason we are doing two organ sites here is that we were quite successful in the prostate cancer field,” says Lars E. Olsson, PhD, Professor of Medical Radiation Physics at Lund University and a physicist at Skåne University Hospital.
As published in Radiation Oncology, the MR-PROTECT trial demonstrated the feasibility of a prostate MR-only radiotherapy treatment workflow using a commercial, deep-learning-based synthetic CT (sCT) generation method (MRI Planner, Spectronic Medical AB, Sweden).1 The team at Skåne University Hospital also clinically validated MRI Planner for sCT generation in the skull.2
“We always had a goal that this synthetic CT method should be used in the clinic,” says Professor Olsson, noting that the aim of the project was to enable MR-only treatment planning of the brain in a clinical setting. In Lerner et al, the authors reported comparable results between sCT and CT images for absorbed dose calculation and patient positioning, enabling clinical implementation of MR-only brain radiotherapy.3 The study was performed using a SIGNA™ Architect 3.0T MR system with the radiation oncology package from GE Healthcare and the MRI Planner software.
Developing an MR-only workflow
The first step toward clinical implementation was to map out the current workflow, pinpoint any minor or major changes that would be required, and then try to foresee any potential implications of these changes. This entire process was conducted in a controlled environment.
“Plan for the unknown,” says Christian Jamtheim Gustafsson, PhD, medical physicist at Skåne University Hospital. “Accept that you don’t know what you don’t know. There are always things that you don’t see from the beginning.”
In a radiotherapy workflow, quality control is crucial. This includes “locking down” the MR acquisition protocols so they cannot be changed. The team also performed an acquisition parameter control prior to the sCT generation to ensure it was precisely the same as when the workflow was validated.
“Quality assurance is a significant part of the MR-only workflow,” adds Dr. Gustafsson.
Specifically, it is possible that an MR scan can lead to geometric distortions, which impacts the quality and precision of radiotherapy planning. To control this, the team used 3D distortion correction and a high receive bandwidth in the acquisition protocol to minimize these effects.
In fact, any distortion detected during the validation process using the GRADE phantom (Spectronic Medical, Sweden) was shown to be small and were of no clinical concern.2
“The geometric distortions were smaller than one pixel and within the recommendations for MR in radiotherapy,4” says Minna Lerner, medical physicist and PhD student at Lund University, which is affiliated with Skåne University Hospital. “Therefore, that spatial accuracy is sufficient such that we would not expect any effect in the brain on the target delineation or the dose calculations.”
Figure 2.
(A) Dose calculations are based on sCT images in the treatment planning system. Evaluation of the dose volume histogram for treatment approval fullfilled the clinical acceptance criteria for all patients. (B) sCT with dose distribution displayed as color wash with a cut-off dose at 10% of the prescribed dose.
There are additional advantages to an MR-only workflow in tumor/target delineation, according to Sara Alkner, MD, PhD, radiation oncologist, Skåne Clinic of Oncology at Skåne University Hospital. First, she doesn’t need to be concerned about the image registration when using only MR images for the delineation. Second, the process is somewhat easier on the patients, as they can avoid scheduling and then undergoing a CT exam.
“Anything we can do to make the process easier for the patient is a big advantage, and that includes skipping a CT exam,” Dr. Alkner says.
Clinical Validation
Twenty-one glioma cancer patients were included in the team’s prospective clinical feasibility study to perform MR-only brain radiotherapy.3 For purposes of the study, all patients underwent a CT to compare to the sCT. One patient was excluded due to the presence of a hemostatic substance injected during surgery, prior to radiotherapy. All participants were treated with volumetric modulated arc therapy (VMAT) with two or three arcs.
As with most brain cancer patients undergoing therapy, daily cone beam CT (CBCT) images were acquired and registered to the CT and sCT images to ensure precise patient positioning. Differences between the CT and sCT were negligible, with a mean and a standard deviation for all patients of 0.3 +/- 0.1 mm (0.1 – 0.6 mm). Differences in dose distributions for both the target and organs at risk, after taking rotations and image resolution into account, were within +/- 1%.
“From all the evaluations we performed, we expect equally good results (for the patients) from the MR-only workflow as with the CT and MR workflow,” Lerner adds. Although a feasibility study does not include outcomes as an end point, she notes the precision in dosimetric and geometric endpoints are equal. The difference, however, when using an MR-only planning workflow is the ability to minimize the treatment margins.
Because sCT is based on Dixon imaging, it efficiently separates air cavities from bone and tissue without prolonged scan times, adds Dr. Gustafsson. “From one five-minute scan, we get the fat, water, in-phase and the out-of-phase signal. This provides a lot of information to the neural net, which provides us with a very high-quality sCT that is correct in Hounsfield units and bone, fat, air cavity, etc.”
Lerner explains, “One of the benefits with MR only is that we exclude the registration uncertainty between CT and MR and in brain cases, that is approximately 2 mm according to the literature.5 So, the spatial uncertainties are reduced with only MR.”
Lessons Learned
While the sCT generation is an important component of the MR-only RT workflow, Professor Olsson says it is a very small part of the entire workflow process. Patient positioning, target delineation and treatment planning will all be impacted. It is also important to implement case-specific QA of the sCT, especially if there are any changes to the MR hardware or acquisition protocols.
“This first validation of an MR-only workflow using sCT in brain gliomas is about ensuring accuracy and building up confidence,” adds Professor Olsson. “The ultimate goal is to take this technique into our clinical routine, as we already have done for prostate cancer.”
As the team prepares to evaluate MR-only stereotactic radiosurgery (SRS) for brain cancer patients, they will again validate the workflow. According to Lerner, 1 mm thick slices will be used for the SRS study, instead of 2 mm that was originally used in the brain validation study.
“We first want to be confident by validating the workflow, which we’ve done, before moving onto other diagnoses and treatment methods,” adds Dr. Alkner.
Now that the team has validated the MR-only workflow for brain patients, they are exploring the potential use of additional MR imaging data in the treatment planning process.
“We may also add other types of MR imaging, for example multidimensional diffusion of brain tumors and metastases that could be part of an MR-only workflow,” says Lerner.
In terms of exploring MR-only RT workflow in other anatomic areas, it is logical to start where both CT and MR are currently used in the simulation and treatment planning process. However, Dr. Alkner notes that MR is being utilized more often in other areas, such as head and neck.
“It is very natural to start in areas where we already rely on MR for target delineation, such as rectal, pelvic and some gynecological tumors. MR is now being used in head and neck cancers, especially when performing curative treatment using radiotherapy prior to surgery, so there could be an advantage for patients,” Dr. Alkner says.
Next for the team at Skåne University Hospital is to evaluate accelerating the MR protocols with AIR™ Recon DL to decrease motion artifacts and increase patient comfort. While there is limited motion in the brain, the fixation mask may be uncomfortable and cause claustrophobia. In the body, such as the prostate, there is internal motion that can lead to image blurring.
“By decreasing the image acquisition time, reduced effects of motion can increase the image quality,” Dr. Gustafsson adds.
Looking back at the success of the MR-only radiotherapy research program, Professor Olsson credits the involvement of the government-funded national program in Sweden. “When there is funding, it is easier to do the research and implement new methods in the clinic. With that funding and the infrastructure, we were able to recruit very skilled physicists and radiation oncologists to the program. Having that close collaboration between research, the PhD students and the clinicians really helped facilitate all the methods and tools that need to be developed for a project such as this.”