Figure 1.
Colonel Dr. Shih with the MAGNUS high-performance gradient MR platform at USU.
A
Figure 2.
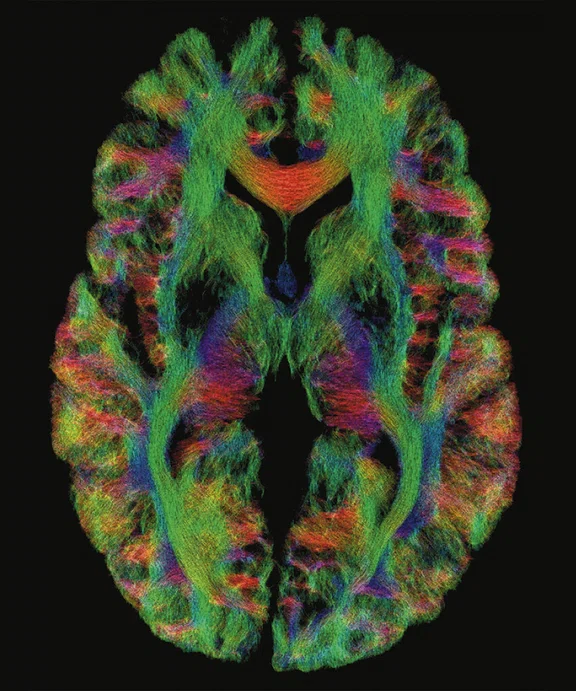
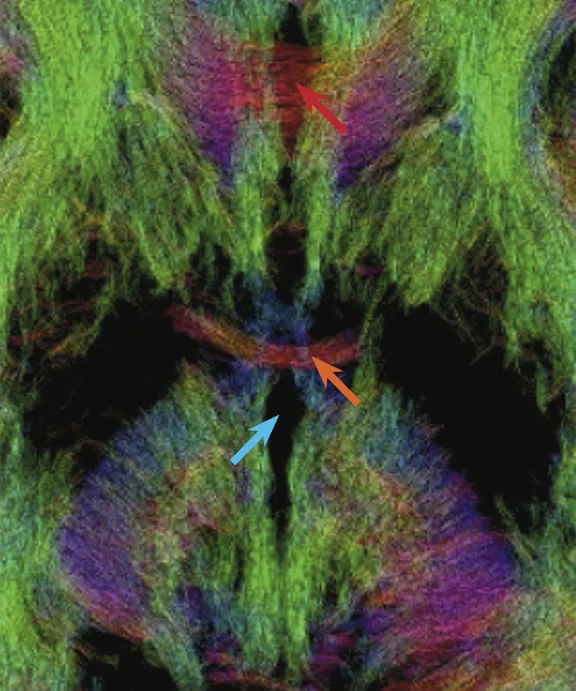
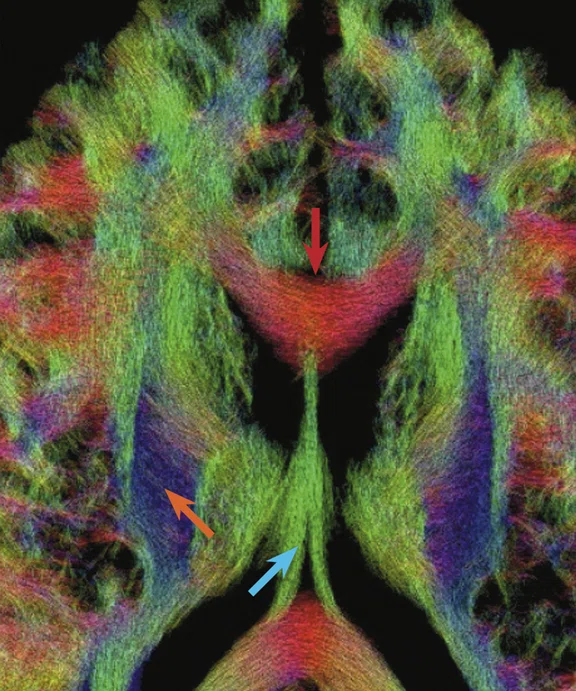
(A) Diffusion tensor imaging at 0.8 mm isotropic resolution on the MAGNUS 3.0T high-performance gradient platform. Acquired with b2000 s/mm2 track density. (B) Magnified image of (A) depicting the corpus callosum (red arrow), anterior commissure (orange arrow) and 3rd ventricle (blue arrow). (C) Magnified image of (A) depicting the corpus callosum (red arrow), corticospinal tracts (orange arrow) and fornix (blue arrow).
B
Figure 2.
(A) Diffusion tensor imaging at 0.8 mm isotropic resolution on the MAGNUS 3.0T high-performance gradient platform. Acquired with b2000 s/mm2 track density. (B) Magnified image of (A) depicting the corpus callosum (red arrow), anterior commissure (orange arrow) and 3rd ventricle (blue arrow). (C) Magnified image of (A) depicting the corpus callosum (red arrow), corticospinal tracts (orange arrow) and fornix (blue arrow).
C
Figure 2.
(A) Diffusion tensor imaging at 0.8 mm isotropic resolution on the MAGNUS 3.0T high-performance gradient platform. Acquired with b2000 s/mm2 track density. (B) Magnified image of (A) depicting the corpus callosum (red arrow), anterior commissure (orange arrow) and 3rd ventricle (blue arrow). (C) Magnified image of (A) depicting the corpus callosum (red arrow), corticospinal tracts (orange arrow) and fornix (blue arrow).
1. Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner’s Guide. Neurochem Res. 2015 Dec;40(12):2583-99.
‡ Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.


result
PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
SPOTLIGHT
The MAGNUS gradient platform: advancing the field of neuroscience to see what could not be seen before
The MAGNUS gradient platform: advancing the field of neuroscience to see what could not be seen before
Disclaimer: The opinions and assertions expressed herein are those of the authors and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences or the Department of Defense. Moreover, references to non-Federal entities or products do not constitute or imply a Uniformed Services University of the Health Sciences or a Department of Defense endorsement.
Concussions, or mild traumatic brain injury (mTBI), can be debilitating and result in chronic symptoms. In certain populations that are more vulnerable to TBI and mTBI, such as athletes and military personnel, these injuries impact not just daily life but the person’s work and career. In many cases, these patients suffer from significant symptoms.
Concussions, or mild traumatic brain injury (mTBI), can be debilitating and result in chronic symptoms. In certain populations that are more vulnerable to TBI and mTBI, such as athletes and military personnel, these injuries impact not just daily life but the person’s work and career. In many cases, these patients suffer from significant symptoms.
For many people suffering from mTBI or TBI, conventional imaging may not detect anything wrong with the brain, which can appear normal on CT or MR.
“If I can’t identify the abnormality in the brain, or measure it, then I can’t make it better,” says Colonel Robert Shih, MD, Chief of Neuroradiology and MRI at WRNMMC and Vice Chair and Assistant Professor of Radiology at the Uniformed Services University of the Health Sciences (USU), Bethesda, MD.
“There are various neuropsychiatric diseases where we hypothesize there is microscopic damage that we cannot detect with conventional imaging,” adds COL Dr. Shih. “There is a good chance that even with a state-of-the-art 3.0T MR we may not see anything. If we do see something, such as nonspecific white matter hyperintensities, we are not sure whether that is causally related to the head trauma.”
Several large studies such as the GE-NFL Head Health Initiative and TRACK-TBI Study (Transforming Research and Clinical Knowledge in TBI) have used advanced diffusion and functional imaging to try to identify mTBI and understand the specific cause of symptoms using 3.0T MR. Although a great amount of data was captured about mTBI, and much knowledge was gained regarding the course of these injuries, the information has mostly informed clinical decision-making at the group level rather than at the individual patient level.
“The limitation we have for identifying mTBI in the individual is really a motivation for us to look at different approaches than what has been done thus far in research,” explains Vincent Ho, MD, Chair and Professor of the Department of Radiology at USU and Director of Research for the Department of Radiology at WRNMMC.
So, the team asked a fundamental question. What if ultra-high performance MR gradients, capable of advanced microstructure imaging, could distinguish patients with mTBI?
MAGNUS‡ (Microstructure Anatomy Gradient for Neuroimaging with Ultrafast Scanning), a collaboration between GE Healthcare, GE Global Research Center and USU, is an advanced gradient MR platform designed to improve diffusion imaging at high b-values without significantly increasing peripheral nerve stimulation or incurring SNR loss. The team designed the MAGNUS gradient system for greater efficiency, resulting in higher maximum gradient amplitude while controlling the inductance to achieve high slew rates with rapid EPI switching and shorter TEs for increased SNR and decreased distortion. “We are starting to see that when we get up to b-values of 35,000 s/mm2, there is no signal from the water outside the axons,” explains COL Dr. Shih. “The only thing left is the water trapped within the axon, so we have a method to directly measure the axon diameter with higher SNR than previously achieved in vivo.”
Research conducted by the National Institutes of Health (NIH) and others have developed mathematical models to disentangle the signal intensity from intra-axonal water and extra-axonal water to estimate individual axon diameters. However, these models do not necessarily correlate with histology.
“With MAGNUS, we can perform high b-value imaging with better SNR than what we could previously achieve,” adds Dr. Ho. “And, it lets us measure intra-axonal diameters directly using a simple signal response model. Prior methods to model axonal diameters have a complicated multi-parameter fitting model.”
The high gradient amplitude and fast slew rate switching capability of the MAGNUS system enables 200mT/m at 500 T/m/s, compared to a conventional 3.0T MR scanner that typically enables up to 80 mT/m at 200 T/m/s. This capability is utilized in an advanced technique called oscillating gradient spin echo (OGSE). As the gradients turn on and off quickly, the frequency of oscillation is varied, which interrogates diffusion over various length scales.
“OSGE allows us to probe the microstructure of the brain in a different way,” Dr. Ho continues. “Now for the first time, because the MAGNUS gradients can achieve high gradient amplitude and slew rate, we can perform in vivo OGSE, which may allow us to identify axon beading in humans, as noted in TBI animal studies using preclinical scanners.”
An abstract presented at the 2022 Military Health System Research Symposium by H. Douglas Morris, PhD, Associate Professor of Radiology at USU and a Visiting Scientist at the National Institute of Neurological Disorders and Stroke of the NIH, demonstrated that an optimized sequence with the higher b-values generated with MAGNUS enabled the reduction of isotropic voxel size from 2.2 mm to 1.5 mm.
“This capability gives us a tremendous advantage for visualizing white matter tracts especially as they radiate out into gray matter,” Dr. Morris says. “With the substantially higher SNR and advanced denoising methods, it is possible to achieve sub-millimeter resolution.”
The rapid switching times allows for shorter echo spacing in EPI readouts, which then minimizes distortions that can occur where the brain interfaces with the skull or paranasal sinuses. The result is better clarity of structures next to the skull base. Dr. Morris hopes to apply this capability to functional protocols to achieve better resolution for functional networks.
“While others have focused on higher field strength, we thought that gradient development was really worth the effort and the impetus to move forward in our line of research,” says Dr. Ho. “It was a fairly significant engineering feat to just build the MAGNUS gradients.”
The unique gradient capabilities and subsequent increase in SNR may also benefit research on the glymphatic system. Recently discovered within the last decade, the glymphatic system is a macroscopic waste clearance system that uses perivascular channels to promote elimination of metabolites and soluble proteins in the central nervous system during sleep. It is believed that glymphatic function is suppressed in brain injuries such as TBI and stroke, and possibly also in neurodegenerative diseases.1 Pre-clinical and human studies have visualized glymphatic flow by injecting a gadolinium-based contrast agent into the subarachnoid space; however, this is not desirable for large cohort studies or routine clinical exams.
Figure 2.
(A) Diffusion tensor imaging at 0.8 mm isotropic resolution on the MAGNUS 3.0T high-performance gradient platform. Acquired with b2000 s/mm2 track density. (B) Magnified image of (A) depicting the corpus callosum (red arrow), anterior commissure (orange arrow) and 3rd ventricle (blue arrow). (C) Magnified image of (A) depicting the corpus callosum (red arrow), corticospinal tracts (orange arrow) and fornix (blue arrow).
“We are investigating if the ultra-high performance gradients will allow us to lower the velocity encoding of phase contrast type imaging to levels where we may be able to detect glymphatic flow,” explains COL Dr. Shih. For example, GE Research and USU have collaborated on and developed an investigational sequence called simultaneous coherent incoherent motion imaging (SCIMI) that has a current velocity encoding (VENC) sensitivity of 0.24 mm/second.
“We cannot achieve a VENC like that on a conventional 3.0T scanner,” COL Dr. Shih adds. “So the MAGNUS gradients are more sensitive for very slow flow.”
There is an ultimate expectation to see the use of high-performance gradients migrate from the bench to the bedside. Even if MAGNUS remains a research tool, COL Dr. Shih believes it will advance the science and understanding of concussion and inform potential treatments. If it becomes an FDA-approved clinical tool, he envisions larger tertiary care and academic centers that have multiple MR systems would be interested in a head-only MR system with ultra-high performance gradients, particularly given the growing demand to image brain injuries and neurodegenerative diseases.
Dr. Ho adds that there are even more patients who could benefit from the ability to image the brain microstructure who have not traditionally undergone imaging for diagnosis and treatment planning, such as those with psychiatric disorders.
Another area of interest is in understanding how humans learn.
“If we can get 3D diffusion imaging down to a spatial resolution of 0.5 mm, then we can actually see the gray-white matter structure in the cortex to better understand how we learn,” says COL Dr. Shih. The implications of this extend to stroke recovery and attention deficit or cognitive disorders, as well as improved diagnosis of progressive diseases such as multiple sclerosis and Alzheimer’s disease. These advanced imaging capabilities may also inform patient selection for drug treatment trials.
“We are in uncharted territory,” adds Dr. Ho. “We have to validate through animal models some of the assumptions that we are seeing in human imaging with MAGNUS, because we are detecting things that we have not seen before.”
Of course, the goal for the MAGNUS research is to inform patient care.
“Can we actually see the individual-level microstructural changes that have eluded us so far, so that we can better classify and stratify patients, provide prognosis and guide treatment?” asks COL Dr. Shih. “We don’t yet have that answer today, but it is a question we are trying to answer.”