A
Figure 2.
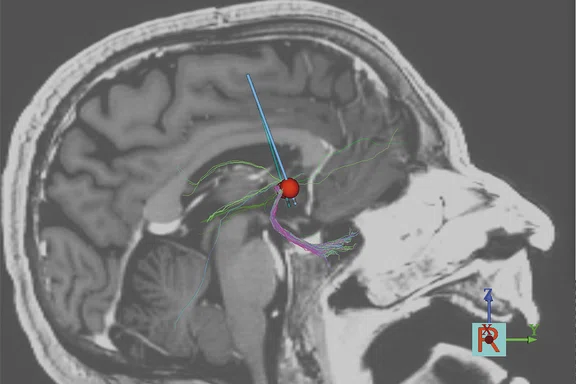
Using tractography models to optimize DBS parameters, it is possible to improve the level of response. (A) Partial response and (B) full response.
B
Figure 2.
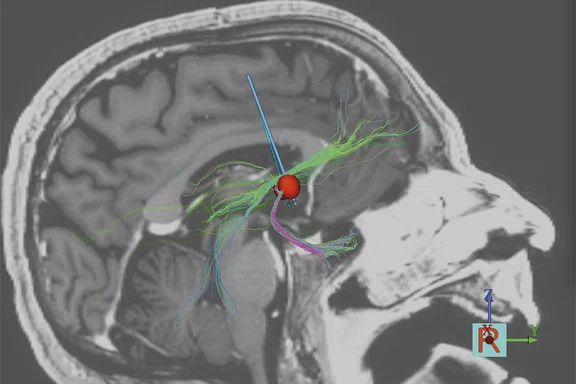
Using tractography models to optimize DBS parameters, it is possible to improve the level of response. (A) Partial response and (B) full response.
1Figee M, Luigjes J, Smolders R, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder [published correction appears in Nat Neurosci. 2014 Sep;17(9):1286]. Nat Neurosci. 2013;16(4):386–387. doi:10.1038/nn.3344
‡Deep brain stimulation devices should be used in accordance with their approved labeling. The indications for use of deep brain stimulation devices may vary by model and by country.
A
Figure 1.
One week prior to surgery, patients are scanned on SIGNA™ Architect 3.0T with a 32-channel phased array head coil. Acquisitions include T1w, T2w and QSM with 64 directions DWI. Post-surgical high-resolution CT data on a Revolution™ Evo system is fused with the MR images. Patient-specific DBS activation volumes (Volume of Tissue Activated: VTA) are modeled using the computational DBS field model developed by Chaturvedi2 and software developed by researchers from Mount Sinai and others (StimVision3). Probabilistic tractography from estimated VTA is performed to identify patterns of white matter connectivity from DBS locations that predict the optimal response.
B
Figure 1.
One week prior to surgery, patients are scanned on SIGNA™ Architect 3.0T with a 32-channel phased array head coil. Acquisitions include T1w, T2w and QSM with 64 directions DWI. Post-surgical high-resolution CT data on a Revolution™ Evo system is fused with the MR images. Patient-specific DBS activation volumes (Volume of Tissue Activated: VTA) are modeled using the computational DBS field model developed by Chaturvedi2 and software developed by researchers from Mount Sinai and others (StimVision3). Probabilistic tractography from estimated VTA is performed to identify patterns of white matter connectivity from DBS locations that predict the optimal response.
C
Figure 1.
One week prior to surgery, patients are scanned on SIGNA™ Architect 3.0T with a 32-channel phased array head coil. Acquisitions include T1w, T2w and QSM with 64 directions DWI. Post-surgical high-resolution CT data on a Revolution™ Evo system is fused with the MR images. Patient-specific DBS activation volumes (Volume of Tissue Activated: VTA) are modeled using the computational DBS field model developed by Chaturvedi2 and software developed by researchers from Mount Sinai and others (StimVision3). Probabilistic tractography from estimated VTA is performed to identify patterns of white matter connectivity from DBS locations that predict the optimal response.
D
Figure 1.
One week prior to surgery, patients are scanned on SIGNA™ Architect 3.0T with a 32-channel phased array head coil. Acquisitions include T1w, T2w and QSM with 64 directions DWI. Post-surgical high-resolution CT data on a Revolution™ Evo system is fused with the MR images. Patient-specific DBS activation volumes (Volume of Tissue Activated: VTA) are modeled using the computational DBS field model developed by Chaturvedi2 and software developed by researchers from Mount Sinai and others (StimVision3). Probabilistic tractography from estimated VTA is performed to identify patterns of white matter connectivity from DBS locations that predict the optimal response.
2. Chaturvedi A, Butson CR, Cooper SE, McIntyre CC. Subthalamic nucleus deep brain stimulation: accurate axonal threshold prediction with diffusion tensor based electric field models. Conf Proc IEEE Eng Med Biol Soc. 2006;2006:1240–1243.
3. Noecker AM, Choi KS, Riva-Posse P, Gross RE, Mayberg HS, McIntyre CC. StimVision Software: Examples and Applications in Subcallosal Cingulate Deep Brain Stimulation for Depression. Neuromodulation. 2018;21(2):191–196.
result


PREVIOUS
${prev-page}
NEXT
${next-page}
Subscribe Now
Manage Subscription
FOLLOW US
Contact Us • Cookie Preferences • Privacy Policy • California Privacy PolicyDo Not Sell or Share My Personal Information • Terms & Conditions • Security
© 2024 GE HealthCare. GE is a trademark of General Electric Company. Used under trademark license.
TECH TRENDS
DTI guides deep brain stimulation intervention in psychiatric patients
DTI guides deep brain stimulation intervention in psychiatric patients
Deep brain stimulation (DBS) is a neurosurgical procedure to implant electrodes in the brain that are connected to a stimulator device for the purposes of treating a variety of neurological conditions. Previously, the technique has primarily been used to treat Parkinson’s disease and, to a lesser extent, epilepsy and essential tremors. At the Icahn School of Medicine at Mount Sinai in New York, Martijn Figee, MD, PhD, Associate Professor of Psychiatry, and colleagues under the direction of Helen S. Mayberg, MD, Professor and Director of the Nash Family Center for Advanced Circuit Therapeutics, are exploring the use of MR-guided DBS interventions in psychiatry‡.
In surgical neuromodulation of psychiatric patients involving DBS, the first and most important aspect is to determine the specific circuits in the brain that are causing the symptoms and disorder. According to Dr. Figee, there is an overlap of the circuits between Parkinson’s and some psychiatric disorders, such as obsessive compulsive disorder (OCD) and depression. In each of these disorders, circuits between basal ganglia and cortical regions orchestrate moving, feeling and thinking in a fluid, efficient way. Specifically, the ventral internal capsule/nucleus accumbens is the most common psychiatric DBS target for OCD.
"Our team has vast experience in DBS for movement disorders targeted at motor basal ganglia circuits. However, little is known about the management of anxiety, depression or apathy in these patients," Dr. Figee says. "In patients with OCD or depression, we are using DBS to modulate the more emotional part of these basal ganglia circuits. We are now trying this also for patients with movement disorders and emotional problems."
At the Nash Family Center, a team of neurologists, neurosurgeons, psychiatrists, neuroscientists and bioinformatic engineers all work together "to advance precision neurosurgical treatments for complex neuropsychiatric disorders through the rapid translation of neuroscience and neuroengineering innovations."
This is where MR imaging – both structural and functional – plays an important role. Using diffusion tensor imaging (DTI), they can identify and measure the circuit connections and white matter tracts for each individual patient (Figure 1). With the introduction of MR-Conditional leads and stimulation devices, challenges associated with imaging implanted patients have significantly decreased along with artifacts from the implanted devices. Scanning at 1.5T has advantages, such as reduced distortion around implants and lower SAR.
Figure 1.
One week prior to surgery, patients are scanned on SIGNA™ Architect 3.0T with a 32-channel phased array head coil. Acquisitions include T1w, T2w and QSM with 64 directions DWI. Post-surgical high-resolution CT data on a Revolution™ Evo system is fused with the MR images. Patient-specific DBS activation volumes (Volume of Tissue Activated: VTA) are modeled using the computational DBS field model developed by Chaturvedi2 and software developed by researchers from Mount Sinai and others (StimVision3). Probabilistic tractography from estimated VTA is performed to identify patterns of white matter connectivity from DBS locations that predict the optimal response.
Prior to the use of DTI and fMRI, the team was using a one-size-fits-all approach when placing the lead or electrode.
"We were always putting the lead or electrode in one place, based on coordinates and an atlas," Dr. Figee explains. "However, we discovered that is not an optimal approach. With DTI, we could see that the tracts run differently in each individual patient."
Before the electrode is implanted, the patient undergoes DTI and fMRI imaging to optimize the neuromodulation intervention. Dr. Figee and his neuroimaging team led by Ki Sueng Choi, PhD, Assistant Professor of Radiology and Neurosurgery, can model which tracts are being stimulated and which ones are being missed.
"When we can be specific to that individual patient, the intervention is greatly improved," he says.
In one study, Dr. Figee and colleagues from the Netherlands compared the signal in the accumbens between healthy individuals and those with OCD who had undergone DBS when they anticipated a monetary reward1. OCD patients will have blunted response (signal) to the reward.
However, OCD patients who were stimulated with the DBS electrode demonstrated a reversal of the blunted response and were normalized compared to the healthy controls. Using SPECT imaging, they could detect a dopamine release in this same region.
"Using these neuroimaging techniques, we were able to show the network changes and that we were modulating a ventral frontostriatal network," Dr. Figee explains.
One of the OCD patients at Mount Sinai was implanted without guidance from DTI. His compulsions remained, although he exhibited less anxiety. By modeling the stimulation with DTI, Dr. Figee found that they were hitting amygdalofugal tracts but hardly any frontostriatal tracts (Figure 2A). After modeling an extra contact on the DBS lead to stimulate these missing tracts, the patient improved dramatically (Figure 2B).
"This case demonstrates how important individualized imaging is to guide our lifesaving decisions."
Dr. Martijn Figee
In a retrospective analysis of responders versus non-responders, Dr. Figee and his previous team from the Netherlands plotted the response toward the most effective tracts to target. The results pointed to the ventral capsule tracts coming from the brainstem. The closer the stimulation was to these tracts, the better the response.
"These tracts have an extremely individualized trajectory, which tells us that we need MR tractography and DTI to individualize the target in each patient and we cannot rely on anatomical MR imaging only," Dr. Figee says. His team performs imaging using DTI with 64 directions on a SIGNA™ Architect with a 32-channel phased array head coil.
"We also intend to use intraoperative MR, which is already in place at Mount Sinai," Dr. Figee adds. "We are testing it as a replacement for CT to image the implanted leads."
The result on patient outcomes has also been significant since moving to connectivity MR imaging. In unpublished work from Dr. Mayberg and her prior team at Emory University with depression patients who did not respond to medication therapy, the group reported an increase in response rates from 40 percent to more than 80 percent since implementing DTI-based targeting.
"This is very inspiring and we hope to move these promising results to a complete response," Dr. Figee says. "By being more specific and individualized in our circuit interventions using DTI and fMRI, we can improve outcomes by making the treatments more effective and predictable. It may also allow us to make these treatments accessible to more patients."